125 Physics Projects for the Evil Genius (72 page)
Read 125 Physics Projects for the Evil Genius Online
Authors: Jerry Silver

Following the previous simulated procedure using pennies, the slope of the line in
Figure 122-2
is 2.7 grams, which is the mass of a single penny. This is a reasonable average for pennies minted before and after 1982. A more precise value can be established by sorting pennies into groups before and after 1982.
The charge of an electron determined by Millikan is –1.6 × 10
−19
Coulombs.
Marbles can be used to simulate the logical process pursued by Millkan in a similar manner that was done with pennies. The marbles have a greater mass, which may make it easier to detect difference. However, finding a relationship graphically may be more difficult because of the variation in mass for a random set of marbles.

Figure 122-2
Using the mass of a penny to simulate the photoelectric effect
.
The size of an oil drop is found by observing its free-fall velocity in air. The oil drop is then given a charge by exposing it to ionizing radiation. The electric field that establishes equilibrium with gravity is related to the force. Although the exact number of electrons on any give oil drop cannot be determined directly, the common multiple leads us to identify the charge of a single electron.
The Millikan oil-drop experiment determines the charge of an electron by measuring the response of an oil drop charged by electrons in an electric field.
Ping-pong ball chain reaction
.
This is a fun and simple demonstration that will help you understand how a nuclear chain reaction occurs. It was used years ago in a Walt Disney film called
Our Friend the Atom
and appeals to all readers including the youngest and technically least sophisticated.
- 24 spring-type mousetraps
- 49 ping-pong balls
- enclosure with at least one transparent side (a large fish tank with glass sides and a glass bottom can work well)
- optional: 2 mirrors the size of, or larger than, the side of the enclosure
1. Set the traps (
Figure 123-1
).
2. Carefully place two ping-pong balls on each of the mousetraps (where the cheese would have gone). See
Figure 123-2
.
3. Lay out the mousetraps in an array that will fit into the enclosure, such as a 6 × 4 array. Obviously, you need to be extremely gentle and avoid sudden motions to prevent a premature release of the mousetrap. Any mishap will likely take other mousetraps out with it.
4. Either lower the enclosure over the mousetraps or develop a way to bring the mousetraps into the enclosure. You may need to experiment with different methods of loading the mousetraps. You may prefer to place the ping-pong balls after, rather than before, moving the traps. You may want to develop a wooden or foamboard template that protects the trap’s trigger mechanism while you are placing the ping-pong balls or glue the traps to a board.

Figure 123-1
Each mousetrap represents a uranium atom
.
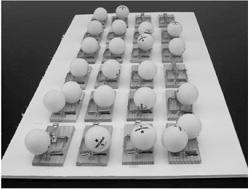
Figure 123-2
Each ping pong ball represents a neutron
.
Expected Results5. With the ping-pong ball loaded on the mousetraps in the enclosure, you are ready to initiate the chain reaction. So far, you have used 48 ping-pong balls, so one should be left. The remaining ball is the neutron that starts the chain reaction.
As in a nuclear-fission chain reaction, a neutron (the starter ping-pong ball) creates the first fission reaction. This event is simulated by the mousetrap releasing two additional ping-pong balls. These, in turn, potentially each release two more balls (neutrons) initiating a doubling of the available neutrons with each fission. As additional ping-pong balls are released, the rate of the reaction accelerates. This chain reaction is simulated by rapidly releasing ping pong balls, which in turn releases other ping-pong balls to continue the reaction. The aftermath of this is shown in
Figure 123-3
.

Figure 123-3
After a simulated chain reaction
.
Nuclear fission occurs in nature when an isotope of a nuclear material absorbs a neutron and become unstable. The nucleus splits, forming two lighter “daughter” nuclei and a spray of free neutrons that produces the cascading effect known as a
chain reaction
. There also needs to be a critical mass for this process to become self-sustaining.
It would be interesting to capture a video image of this simulated nuclear reaction and view it in slow motion.
Nuclear fission is initiated by a free neutron that causes a nucleus (such as a uranium-238 nucleus) to split and release additional neutrons. This is the basis of nuclear power, which currently provides about one-fifth of the electricity in the United States.
The sodium doublet. Why do we think the electron has both up and down spins
?
No one has ever seen an electron spin. In fact, for that matter, no one has ever even seen an electron. Yet, we know an electron behaves as
if
it were spinning. Some of the most revealing evidence for this comes from the light that certain atoms emit when they’re excited.
If some sodium chloride is exposed to a flame, the flame takes on a characteristic yellow/orange color. This is the color observed in the common flame test used in chemistry labs to identify the presence of sodium in sodium vapor street lamps. If you look at the light coming from an excited sodium atom with a spectroscope or diffraction grating, the first thing you notice is a single orange/yellow line with a wavelength between 589 and 590 nanometers.
However, on closer inspection, you notice
not one but two
orange/yellow lines. The purpose of this project is to observe these two lines, known as the
sodium doublet
, and, more importantly, to understand why they are split.
- Bunsen burner or other flame
- concentrated sodium chloride solution
- clean nichrome wire loop (or a wooden splint)
- diffraction grating or spectroscope
- sodium vapor discharge tube with appropriate high-voltage power supply
- Use one of the previous methods to produce a light source generated by excited sodium atoms.
- Darken the room.
- Observe the light using a diffraction grating or a spectroscope.
- Look carefully until you see a vertical yellow/orange line. Look closely until you notice this line is formed by two separate lines. See
Figure 124-1
.
The point of this project is to observe two separate yellow/orange lines that make up the sodium doublet.
When an electron goes from one energy level to a lower energy level, it gives off light. Each energy level can hold two electrons: one with spin up and the other with spin down. The electron with the spin up takes a slightly greater amount of energy to go from one energy level to another. As a result, the electrons with different spin conditions give off a slightly different color (wavelength) light.
Continuing the New Jersey Turnpike analogy (from
Project 120
), let’s say you travel a certain distance going from Exit 7 to Exit 8. But things are slightly different if you get off at either an eastbound or westbound ramp at the exit. That small difference can be thought to be something like the effect caused by electron spin.

Figure 124-1
Electrons in a sodium atom produce a primary characteristic wavelength when electrons move from one energy level to another. A slightly different wavelength is produced depending on whether the spin of the electron is “up” or “down.”
If an excited sodium atom is exposed to a very powerful magnetic field, these spectral lines split even further. This is called the Zeeman effect, which requires magnetic fields on the order of 18 Teslas. However, because this is roughly 20 times more powerful than the very strong magnetic fields used to study nuclear magnetic resonance, we won’t pursue Zeeman splitting experiments in this book.
In an atom, electrons have up or down spin. When an electron goes from one energy level to another, the energy given off by each of the two spin orientations is slightly different. Observing the split in the frequency supports the concept of electron spin.