Arrival of the Fittest: Solving Evolution's Greatest Puzzle (10 page)
Read Arrival of the Fittest: Solving Evolution's Greatest Puzzle Online
Authors: Andreas Wagner

Part of the reason why
E. coli
’s metabolism is so complex lies in the sixty-odd biomass building blocks. Manufacturing each of them requires multiple reactions and intermediate molecules. Another part is that
E. coli
is a phenomenal survivor, thriving not only on the rich nutrient broth of our guts but also in an austere nutrient desert where only seven small molecules supply chemical elements and energy. This minimal environment is so spartan that one molecule like glucose does double duty as a source of both energy and the chemical element carbon. From these few ingredients,
E. coli
can manufacture everything it needs, all sixty-odd biomass building blocks, and from them, the entire cell.
But that’s not all. You can remove glucose from a minimal chemical environment and replace it with another source of carbon and energy, such as glycerol.
E. coli
can still build its body from the carbon and energy in this molecule. Replace glycerol with the acetic acid in vinegar and, again,
E. coli
can build its body. All in all,
E. coli
can use more than eighty different molecules as its
only
source of energy and as its only supplier of every single one of the billions of carbon atoms in its cells. It is similarly flexible about other elements, such as nitrogen and phosphorus.
E. coli
is like a self-building, self-multiplying, self-healing race car that can run on kerosene, Coca-Cola, or nail polish remover.
Simple chemical environments are useful for studying microbes in the laboratory, but they are rare in the wild. An environment like the soil or the human gut contains dozens of ever-changing fuel molecules. To harvest energy and to extract building materials from these molecules require a distinct sequence of chemical reactions for each of them. And to make a good living, a microbe must be able to exploit all of them.
Suddenly, a thousand reactions don’t sound like a lot.
Another difference between today’s life and its shadowy ancestors lies in the catalysts, those molecules that accelerate chemical reactions. If your gut did not contain the right catalyst—an enzyme known as
sucrase
—the sucrose in a drink of sugared water would take years or decades to split into glucose and fructose.
66
You could drink gallons of sugared water every day, and starve to death.
Reactions like this are no longer accelerated by the simple metal-containing mineral catalysts of early life. Modern catalysts speed up some reactions a trillionfold, allowing molecules to react as soon as they meet. Each of these molecular machines—and there are several thousand of them—is a specific string of amino acids.
67
The enzyme
sucrase
, for example, is a gigantic molecule with 1,827 amino acids, each of them with at least a dozen atoms, adding up to twenty thousand atoms per sucrase molecule.
68
The table sugar sucrose with forty-five atoms is minuscule by comparison—like a pea compared to a football—which explains why enzymes are called
macro
molecules, as opposed to the small molecules they help react and the biomass building blocks they help construct.
69
Sucrase may seem large, but it is not even unusual. Many enzymes are much larger.
While the sucrase string is manufactured, it curls and twists in three dimensions, like a ball of wool, but with important differences: Every ball of wool is unique, but every sucrase molecule is the same. As sucrase is manufactured, it folds in space in a precisely stereotypic manner. What is more, folded sucrase is constantly wiggling, jiggling, and vibrating to perform its catalytic duty. Think of sucrase as a self-assembling nanomachine whose movement is so fast it would be a mere blur, taking molecules in, cleaving them, and spitting out their products at lightning speed.
Every cell contains thousands of such nanomachines, each of them dedicated to a different chemical reaction. And all their complex activities take place in a tiny space where the molecular building blocks of life are packed more tightly than a Tokyo subway at rush hour. Amazing.
We do not yet know how life evolved all this complexity from its simple origins, and we may never know for sure. The oldest single-celled fossils are as complex as modern cells, and their ancestors are shrouded in darkness. This should come as no surprise. The eons have ground away most ancient rocks, and even if the churning continents had not liquefied their remnants, early life was a fragile bag of molecules. It was nothing like the sturdy mats of blue-green algae—more correctly called cyanobacteria—that left behind 3.5-billion-year-old calcium imprints known as stromatolites, and even less like the big-boned dinosaurs who lived a relatively recent hundred million years ago.
We do know, however, that we all come from a single common ancestor. This is not the same as saying that life originated only once. Given the powers of self-organization, I would not be surprised if life arose many times, in hydrothermal vents, in warm ponds, or who knows where else. Among a multitude of faint lights that flickered on and off throughout the earliest history of the planet, some held steady, while others shone more and more brightly. But only one of them became bright enough to spawn all of today’s life. This is not a matter of opinion. It has to be true, for a single reason: standards. More accurately,
universal standards.
The computer scientist Andrew Tanenbaum once quipped, “The nice thing about standards is that you have so many to choose from.”
70
I know what he was talking about. Whenever a remote control, a clock, or some other gadget stops working in my home, I rummage through a cabinet in my living room that contains a zoo of batteries large and small—but usually not the right one. Life would be easier if it offered only one kind of battery. Or one kind of coffee filter, data storage medium, or computer operating system. Even old technologies suffer from this problem: After more than a century of public electric power, fourteen incompatible outlet standards exist around the world, a curse for millions of international travelers who arrive in foreign countries every day accompanied by laptops, hair dryers, electric razors—and the wrong outlet adaptors.
Nature is different. It has standardized energy storage. Among the many forms that energy can take, such as mechanical (a wrecking ball smashing into a house), electrical (the current of electrons powering a computer), or chemical (the bonds that tie atoms together in a molecule), chemical energy is life’s favorite. All organisms on the planet, from single-celled bacteria to the blue whale, use a standard means to store energy, the molecule adenosine triphosphate (ATP). When its energy-rich chemical bonds rupture, energy is transferred to other molecules, and the less energy-rich molecule adenosine diphosphate (ADP) is created. To regenerate the energy-rich ATP, specialized enzymes can transfer energy to ADP from fuel molecules.
Not all of ATP’s chemical energy ultimately ends up in other molecules. Bacteria use ATP to power the tiny whirring flagellae that propel them through water. Fireflies use ATP to illuminate their bodies when they hope to attract mates. Some eels transform ATP into electrical energy that dispatches prey with powerful electric shocks. But regardless of its final form—mechanical, light, electric—the energy in living things ultimately comes from the chemical battery of ATP.
When a cell uses a chemical fuel like glucose to manufacture one of the cell’s biomass building blocks, it first converts the chemical energy from glucose into the chemical energy of ATP. It then uses ATP’s chemical energy to build, step by step, the chemical bonds of the building block. In this way, the energy stored in the fuel eventually ends up in the bonds of the building block. ATP is a crucial middleman in this energy transfer.
Living things have adopted ATP as the universal energy storage standard—no rummaging for batteries or paying a premium for an airport power adapter.
71
Every organism living today can trace its descent from the inventor of life’s most successful power storage innovation. And power storage is not life’s only standard. We have already encountered the ancient heart of metabolism, the citric acid cycle, and the universal membrane molecules with their love-hate relationship to water.
72
And let’s not forget DNA, RNA, and the genetic code that translates triplets of DNA letters into amino acids—a code understood by all organisms.
73
ATP and the citric acid cycle aren’t universal standards in the same way that the speed of light is a universal speed limit. They aren’t the
only
way to build life. We know alternatives to our genetic code, to ATP as an energy carrier, and even to DNA as an information repository.
74
Life’s standards are the historical legacy of a single ancestor. The marathon that started at life’s origins may have begun with many hopeful participants, but whether through natural selection or dumb luck, only one crossed the finish line to leave its descendants today. This is a bit depressing, if you extrapolate from the present to your chances of leaving descendants in the distant future. But it also contains a hopeful message, at least for frequent travelers: Wait another four billion years, and you may not need an outlet adapter.
By the time you read these lines, the puzzle of life’s origin may be complete. We may know whether life began in a warm pond, in a hydrothermal vent, in a freezing ocean, or in outer space. Or we may have to wait another century. But more important for understanding innovability than reconstructing the one true scenario are two general lessons that all scenarios have in common.
The first is that life needed to innovate even before it became life—by creating the first autocatalytic metabolisms and the earliest replicators.
The other is that life’s symphony of innovation has three major themes. First, innovations created new combinations of chemical reactions, such as those that form life’s building blocks and that built the first replicators. Second, innovation required molecules that could help other molecules react. Third, innovation created new regulation, the key to coordinate complex life. These three themes resounded louder and louder in the biosphere as life became more and more complex and innovability increased. Primitive metabolism has grown into a giant network in which chemical reactions are combined and recombined to permit life’s expansion into every conceivable habitat. Sophisticated protein molecules have pushed aside simple inorganic catalysts, and have given rise to innovations as different as light-detecting opsins and armor-providing keratins. And regulation, a seemingly mundane process, has become an innovation industry all by itself, bringing forth multicellular organisms with limbs, a heart, and a brain.
From the origin of life to today, innovations have been transforming metabolism, proteins, and regulation. And although the three seem very different, a curious but powerful kind of self-organization stands behind their ability to innovate.
CHAPTER THREE
The Universal Library
I
magine standing in a room crammed with books from floor to ceiling. The bookshelves barely leave space for the door you see on each of the four walls. You start leafing through the books and realize that they all have the same number of pages. Each page contains the same number of lines. And each line has the same number of characters. But—this is strange—the books are full of gibberish. Each line of each page of each book contains mostly arbitrary strings of letters—“hsjaksjs . . . ,” “zvaldsoeg . . . ,” and so on—occasionally separated by spaces and punctuation. Only rarely do you find a meaningful English word—“cat,” “teapot,” “bicycle”—islands in a vast sea of more gibberish.
After a while you tire of these books, which do not make sense. You step through one of the doors and find yourself in another room just like the first one. It is equally packed with bookshelves that crowd in on four doors. And its books make no more sense than those in the first room.
Another door leads you to yet another identical room, and from there you begin to wander through room after room after room, and realize that you are in an endless maze of rooms, identical except for the books that inhabit them. These books form a library that is as gigantic as it is bizarre.
1
As you wander through this library, you encounter fellow travelers who help you grasp the enormity of this place.
The rooms form a universal library, home to all conceivable books.
That is, its books contain all possible strings of characters—twenty-six letters and a few punctuation marks. Most of the strings are the nonsense you already read. But occasionally a book will contain a meaningful word, sentence, or paragraph. More than that, somewhere in this library dwell books that contain no gibberish whatsoever. Because the library contains all possible books, it also contains each meaningful book ever written. All possible novels, short stories, poetry collections, biographies (of people real or imagined), philosophical treatises, religious books, books of science and mathematics, all conceivable books written not only in English but in all languages, books that reveal everything that is true, but also spin terrible lies, books that talk about other books, about the library itself and where it came from, books, some true, others false, about your life’s story, how it began and how it will end, and the book you are reading right now. All of them are contained in this library—a library enormous almost beyond imagining.
To get of an idea how large this library is, let us say that every book in it contains 500,000 characters. (That’s not very long—in the same ballpark as the book you are reading right now.) Excluding punctuation marks, there are 26 possibilities (A through Z) for each of these 500,000 characters. That is, there are 26 possibilities for the first character, 26 for the second, 26 for the third, and so forth. To estimate the number of books, we thus need to multiply 26 by itself 500,000 times. Mathematicians would write this number as 26 raised to the power of 500,000, or 26
500000
. This is a very large number, amounting to a 1 with more than 700,000 zeroes behind it, more zeroes than this book has letters. And far greater than the number of hydrogen atoms in the universe. It is a hyperastronomical number.
The deepest secrets of nature’s creativity reside in libraries just like this: all-encompassing and hyperastronomically large. Only instead of being written in human language, the texts in these libraries are written in the genetic alphabet of DNA and the molecular functions that DNA encodes.
Human books can capture entire universes—everything that human language can utter—but they have nothing on the chemical language of what may be life’s oldest library of creation, the one devoted to metabolism. Every one of the trillions of living things on earth can be
described
by human prose or poetry. But
creating
any one of them requires the chemical language of metabolism, the chemical reactions that create the building blocks of life and thus ultimately all living matter. The library’s chemical language can express life itself—all of it.
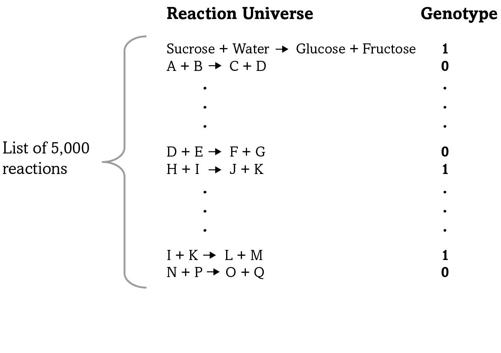
FIGURE 4.
A metabolic genotype
To date, we have discovered more than five thousand different chemical reactions that some organism, somewhere on our planet, uses to produce the building blocks of life I mentioned in chapter 2, the nucleotides that make up DNA and RNA, and the amino acids from which proteins are constructed. The reactions that occur in
E. coli
—more than a thousand—are among them, as are all known chemical reactions that take place in any bacterium, fungus, plant, or animal—including humans. When your body extracts energy from sugar or any other food, it uses such reactions. It also uses them when healing the few hundred skin cells covering a scraped knee, and when replenishing the millions of red blood cells that die every day.
No organism can catalyze all five-thousand-odd known reactions, but every organism can catalyze
some,
and the reactions it
can
catalyze make up its metabolism. For multiple organisms we know these reactions, thanks to twentieth-century biochemistry and to the technological revolutions of the early twenty-first century. They gave us access to a mountain of metabolic information on more than two thousand different organisms, stored in giant online repositories, such as the Kyoto Encyclopedia of Genes and Genomes, or the BioCyc database, and accessible in split seconds from any computer with an Internet connection.
2
Figure 4 shows how we can organize this information. The left side of the figure stands for a list of five thousand reactions—written as chemical equations. To avoid clutter I wrote out the molecules in only one of them—the sucrose-splitting reaction—but simplified all others to a single letter. Let’s consider one organism, such as
E. coli
or a human, and mark a “1” next to a reaction if our organism can catalyze this reaction—it has a gene making an enzyme for it. Otherwise we’ll mark a “0.” The result is a long list of ones and zeroes like that in the figure, a compact way to specify a metabolism.
Bacteria such as
E. coli
can make all twenty amino acids in proteins, whereas metabolic cripples like us humans can make only twelve of them. We lack the necessary enzymes and reactions for the remaining eight. The figure’s shorthand way of describing a metabolism is ideal for expressing differences like this: Because we lack some reactions, our list of reactions contains some zeroes where that of
E. coli
contains ones.
A list like this is also an extremely compact way to write an organism’s
metabolic genotype
—the part of the genome encoding its metabolism—because an organism’s list of reactions is ultimately encoded in its DNA. You can also think of the list as a text written in an alphabet with only two letters, and without spaces or punctuation marks, like this: “1001 . . . 0110 . . . 0010.” The first letter in such a text might correspond to the sucrose-splitting reaction, which is present (“1”) in this example, whereas the second reaction might be one of those needed to synthesize an essential amino acid—it is absent (“0”) in this example text but could be present (“1”) in another organism’s genotype—and so on.
It is a text in a library vast beyond imagination, the library of all possible metabolisms.
The number of texts in that library can be calculated with the same arithmetic that computed the size of the universal library of books. Because each reaction in the known universe of reactions can be either present or absent in a metabolism, there are two possibilities (present or absent) for the first reaction, two for the second reaction, and so on, for each reaction in the universe. To calculate the total number of texts, we multiply the number 2 by itself as many times as there are reactions in our universe. For a universe of 5,000 reactions, there are 2
5000
possible metabolisms, 2
5000
texts written in the alphabet of zero and one, each of them standing for a different metabolism. This number is greater than 10
1500
, or a 1 with 1,500 trailing zeroes. While not quite as large as the number of texts in the universal library of human books, it is still much larger than the number of hydrogen atoms in the universe. The metabolic library is also hyperastronomical.
And just as the universal library contains all meaningful books, the library of metabolisms contains all “meaningful” metabolisms—those that allow an organism to survive—and many more, because not all metabolisms are meaningful, just as not all books are. Some metabolisms cannot procure energy, or they fail to manufacture important molecules. These are like books where some chapters, paragraphs, or sentences are coherent but the book as a whole does not make sense. And many other metabolic texts are gibberish. These are metabolisms with disjointed reaction sequences dead-ending on molecules useless to life, the equivalent of books containing only meaningless character strings.
If you wandered through the universal library of books long enough, you would find books that surprise you. They contain novel thoughts, ideas, and inventions. The genotypic texts in the universal metabolic library are no different. They can encode metabolisms with never-before-seen chemical abilities, novel phenotypes that manufacture new molecules or use new fuels. In short, innovations.
Because metabolism is as old as life itself, evolving life has explored this library ever since it originated. A billion years ago, nature had already discovered unimaginably many metabolic phenotypes, enough of them that it might have stopped finding innovative metabolic texts long since. But far from resting on its early laurels, evolution is still discovering such texts, much faster than we can decipher them, in billions and trillions of organisms alive today. Some of these texts appeared less than a hundred years ago—a mere moment in evolutionary time.
Consider pentachlorophenol, a nasty molecule that humans first produced in the 1930s. It is used in antifouling paint to coat ships’ hulls, and also as an insecticide, fungicide, and disinfectant—in short, to kill life. Pentachlorophenol also damages our kidneys, blood, and nervous system, and it causes cancer. But despite its noxious nature, life has found ways not only to tolerate pentachlorophenol but to thrive on it. The aptly named bacterium
Sphingobium chlorophenolicum
can extract both energy and carbon from it, using pentachlorophenol as its only food source. To do so, its genome encodes four enzyme-catalyzed reactions that convert pentachlorophenol into molecules that are as digestible as glucose—the equivalent of transforming a chemical weapon into a chocolate bar.
3