Read Arrival of the Fittest: Solving Evolution's Greatest Puzzle Online
Authors: Andreas Wagner
Arrival of the Fittest: Solving Evolution's Greatest Puzzle (16 page)

After analyzing thousands of network pairs, and after studying phenotypes involving eighty different fuel molecules, we had found that the premise was correct. Different neighborhoods contain texts with new meanings, but these meanings differ between neighborhoods. Most metabolic innovations are unique to one neighborhood and do not occur in the other. (Because each new phenotype has its own genotype network, this also means that different genotype networks in the library are interwoven in an unfathomably complex way.)
We then went one step further. With our computers’ help, we wandered once again through a genotype network in the metabolic library, except that now we behaved like inventory clerks with their notepads, listing all the innovations in the immediate neighborhood of our path—all innovations that were within easy reach. We listed all the different new phenotypes in the walker’s neighborhood before the start of the walk, and examined the neighborhood again after the first step. If it contained a new phenotype that was not already on the list, we added it to the list, took one further step, examined the new neighborhood, added any new phenotypes, and so on, for thousands of steps. Because we knew that different neighborhoods contain different innovations, we expected the list to grow over time, as new phenotypes became accessible. But we expected that we would run out of new phenotypes eventually.
Wrong. Long after our notepads were full, we were still encountering innovations.
Worried that this trip had yielded an unusually rich bounty, we went on many more trips, from different starting points in the library, metabolisms viable on different fuel molecules. And we also crowdsourced our shopping, exploring the library not with a single metabolism but with entire populations of evolving metabolisms to tally how many different new phenotypes they found. In every instance, innovations continually piled up, with no sign of slowing down, at a steady clip, unceasingly, no matter how long the exploration continued, a hundred, a thousand, or ten thousand steps, hours, days, weeks, until we ran out of time and needed to do other work. We realized that the innovability of an evolving metabolism would not exhaust itself in our lifetime.
47
Innovability in the metabolic library is near limitless, and for that both genotype networks
and
diverse neighborhoods are required. They are the two keys to innovability. Genotype networks guarantee that evolving populations can explore the library. Without them the lethal punishment of losing viability would be inevitable. But without diverse neighborhoods in this library, exploring a genotype network would be pointless: The exploration would not turn up many texts with new meanings.
Any librarian who wanted to organize a human library in this way would be locked away. Even if a thousand books told the same story in different ways, no sane librarian would create sections that placed books with all manner of different meanings next to one another. And he would certainly not pack
different
neighborhoods around synonymous texts with books in
different
subject categories.
But a closer look reveals that the metabolic library’s catalog is far from a madman’s febrile fantasy. Human libraries are useful only because we
have
librarians who make catalogs suitable for us, where books on photovoltaics stand on one shelf, those on French literature on another, and so on. For a library whose readers have no catalog and can only take random steps, and where missteps are punishable by death, it would be disastrous, because they would be stuck on whatever shelf they started. They would be idiots savants, world experts in one area but completely ignorant in all others, and could never learn anything new—not a smart strategy for surviving in an ever-changing world. For such readers, the metabolic library is perfect, uncannily well set up for innovation. It guarantees eternal learning and innovability.
Even more uncanny: Life’s other libraries are organized the same way.
CHAPTER FOUR
Shapely Beauties
T
he Arctic cod is a slender, brownish fish, with a silver belly and black fins, between eighteen and thirty centimeters long, a perfectly unremarkable occupant of the world’s oceans. Except for one thing: The Arctic cod—
Boreogadus saida
—lives and thrives within six degrees of the North Pole, nine hundred meters beneath the surface, in waters that regularly chill below zero degrees Celsius.
At that temperature, the internal fluids of most organisms turn into ice crystals with edges as beautiful as well-forged swords, and just as deadly, for they carve up living tissue like butter. Warm-blooded animals have a built-in thermostat that allows them to survive in subfreezing weather. Fish don’t. And yet, there’s the Arctic cod.
B. saida
survives by producing antifreeze proteins that lower the freezing temperature of its body fluids, much like the antifreeze in a car’s engine coolant. These proteins are prototypical examples of nature’s innovative powers. Change the amino acid sequence needed to produce a particular protein and, presto, huge areas of the earth’s oceans become livable.
1
Antifreeze proteins are among thousands of innovative wonders that populate the cells of fish and of every other living being. If you could shrink yourself and travel through a cell, you would first be astonished by how many
different
kinds
of molecules there are, millions of them. Tiny molecules like water, larger molecules like sugars or amino acids, and even larger macromolecules like proteins all push and jostle and shove past each other like subway commuters during rush hour.
Proteins, the giant hulking monsters of a cell’s molecular population, are life’s workhorses. We have met the metabolic enzymes that synthesize everything a cell needs—including their own amino acids—by linking smaller molecules, cleaving them like molecular scissors, or simply rearranging their atoms.
2
But not all proteins are enzymes. Some are molecular motors, like the proteins that help your muscles contract, or like the
kinesins
that “walk” along stiff molecular cables that crisscross the cell, carrying tiny membrane vesicles that shelter various molecular cargoes. Mayhem ensues when these truckers of the cell no longer do their job. One kinesin, for example, transports building materials needed to wire the cells in our nervous system, and mutations in its gene can cause an incurable disease called Type 2A Charcot-Marie-Tooth disease, which hampers movement and sensation in feet and hands.
3
Yet other proteins attach to DNA and switch genes on or off. These
regulatory
proteins allow the information encoded in a gene to become transformed into an amino acid string. Hundreds of such regulators work simultaneously, each of them flipping the switch on some genes but not on others. (They are the source of yet another kind of innovation we will explore in chapter 5.)
And there’s more: rigid protein rods that form a cell’s molecular skeleton, proteins that import nutrients, proteins that dump waste outside the cell, proteins that relay molecular messages between cells, and on and on.
Each of these proteins has its own special talent, expressed in its phenotype, whose most important aspect is shape.
4
I do not just mean the molecular shape of the twenty kinds of amino acids in proteins, and the order in which they are strung together—the
primary structure
of a protein.
5
I mean the shape this string forms in space through the protein folding process that I first mentioned in chapter 1.
Hydrophilic
amino acids
love to be near the water that surrounds them, whereas
hydrophobic
amino acids
avoid water—like the oily parts of membrane molecules—and these molecular sympathies help an amino acid string fold in a stereotypical manner. Driven by heat’s vibrations, a folding protein explores many shapes of its amino acid chain until it finds one where most water-avoiding amino acids cluster together and form a densely packed core that is surrounded by the water-loving molecules on the protein surface.
6
What’s more, some amino acids attract and others repel each other, and these chemical sympathies also influence a protein’s fold. The protein folding process—driven by nothing but erratically bouncing molecules—is yet another reminder of the power of self-organization. It occurs millions of times a day in each of our trillion cells, whenever they manufacture a new protein string.
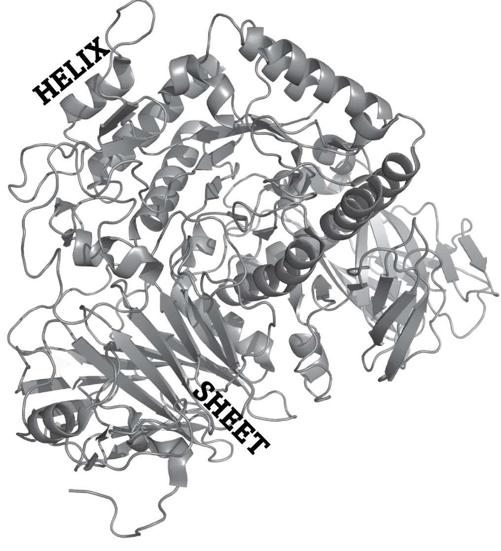
Viewed atom by atom, the three-dimensional
fold
of a protein like the sugar-splitting sucrase from chapter 2 would appear like a shapeless blob. But by stepping back and focusing on the string that holds the amino acid beads together (figure 10), one can discern regular and repeated patterns of amino acid arrangements in space that occur in many proteins. They include helixlike corkscrews—one is labeled in the figure—or several parallel strands, an arrangement also called a
sheet.
7
Helices and sheets are major elements of protein folds and form the
secondary structure
of a protein. And these helices and sheets, together with the strands that connect them, form the labyrinthine three-dimensional or
tertiary structure
of a protein’s fold shown in figure 10.
FIGURE 10.
Sucrase folded in space
Even though it may look like a tangled pile of spaghetti, the fold in figure 10 is actually highly organized: Any two sucrase amino acid strings spontaneously fold into the exact same shape.
8
This shape is critical to a protein’s function, because its helices and sheets guide and constrain the endless heat-induced vibrations and oscillations of the folded protein. Such constrained movement allows enzymes like sucrase to cleave sucrose—a bit like the blades of scissors whose movement is constrained by the pivot that connects them and enables them to slice paper.
9
Because heat-caused vibrations are so important to enzymes, these molecules also have an optimal temperature: Too little heat and their vibrations are not powerful enough to reorganize molecules. Too much heat and the vibrations shake the fold apart—into the linear string of amino acids. Worse than that, unfolded proteins often aggregate into large inert clumps like the proteins in boiled eggs. Such clumps of unfolded proteins are more than useless: When too many of them accumulate, for example in your brain, they bring forth horrible diseases like Alzheimer’s.
10
In the bewildering realm of oscillating shapes that sucrase and other proteins inhabit, each shape has a specific job. Each is well suited for what it does and highly complex. In the words that Darwin used to describe the living world, it is a world of “endless forms most beautiful,”
11
but
these
forms—unknown to Darwin—keep the living world alive.
Proteins do not just perform existing jobs. The economy of living organisms, like that of the human world, is constantly changing, and in response, evolution brings forth new protein shapes, innovations that take on new jobs. These jobs open whenever life needs to solve a new problem, like that of surviving the menacing knives of growing ice crystals.
And just as in the human economy, where inventions from blast furnaces to smartphones are often made several times independently, the innovations that fill these jobs are often discovered more than once. Antifreeze proteins are a case in point: They originated not just in the Arctic cod but also in Antarctic fish, and from different proteins in their ancestors.
12
They even originated more than once in the Arctic.
13
What’s more, some fish evolved more than one kind of antifreeze protein. The winter flounder, a flatfish from the North Atlantic, manufactures one antifreeze protein that prevents its bloodstream from freezing, and another that protects its skin.
14
And some of these proteins arose very quickly in evolutionary terms—in less than three million years.
Dozens of amino acids had to change from proteins in some frost-sensitive ancestor to create antifreeze proteins, but protein innovations often require much less change.
15
Alter as little as one amino acid in the enzyme needed to manufacture the amino acid histidine, and the result is a new enzyme that helps manufacture the amino acid tryptophan.
16
Mutate a specific amino acid in an
E. coli
enzyme that helps extract energy from the sugar arabinose—its name comes from gum arabic, a natural gum from acacia trees—and this enzyme transmogrifies from a rearranger of atoms to a cleaver of molecules.
17
Such minimal changes can have dramatic consequences for life, as the bar-headed goose from Central Asia could tell us. It is one of the world’s highest-flying birds. It has to be, since its migratory route takes it across the Himalayas at altitudes that exceed five miles, where the air is not only thinner—requiring birds to flap harder—but contains only a third as much oxygen by volume as sea-level air. At that altitude, the mountaineers who struggle up Mount Everest use oxygen tanks, and the passengers on jet airliners require pressurized cabins. The goose can’t benefit from either technology, but no problem, it has an even better trick. Its hemoglobin—the protein that shuttles oxygen from lungs to muscles—harbors an amino acid change that helps it bind oxygen much more tightly than our hemoglobin. It allows the goose to scavenge oxygen molecules from thin air, and keeps this bird flying where others are grounded.
18
Molecular innovations like the Arctic cod’s antifreeze or the bar-headed goose’s oxygen-binding hemoglobin are valuable because they expand an organism’s habitat, which means more food, better survival, and more offspring. Other innovations confer a different kind of advantage, such as the ability to discriminate between one kind of food and another, to choose a nutritious rather than a poisonous plant for dinner. They depend on improving perception, rather than mobility, and they are why the retina in the back of our eye contains three kinds of opsins. These are highly specialized proteins that detect light and are tuned to the different wavelengths of blue, red, and green light. Thanks to them, we see the world in color. This was not always the case. Our most distant ancestor among the vertebrates probably had only one opsin. Theirs was a black-and-white world. Most mammals have two different opsins, those for red and blue. They can see in two colors. But we and some of our relatives like chimpanzees can see in three colors, perhaps because color vision helped our distant ancestors forage: It lets fruits stand out from the background of green foliage. Whatever the reason, the innovation of color vision takes very little change, as little as three altered amino acids that retune an opsin from red to green.
19
Innovations like color vision benefit us, but others harm us—those of deadly bacteria that resist the antibiotics your doctor prescribes. They are the unfortunate side effect of our continual improvement of antibiotics, the result of a biological arms race of bacteria against biotechnologists. This race evokes the Red Queen of Lewis Carroll’s
Through the Looking Glass,
who famously told Alice, “Now,
here
, you see, it takes all the running
you
can do, to keep in the same place.”
20
Through it, bacteria have discovered various protein innovations, some of which destroy antibiotic molecules, whereas others, known as
efflux
pumps, force antibiotics out of the cell like some bacterial rescue squad pumping toxic gas out of a contaminated house. (Horizontal gene transfer, combined with human travel, can spread such innovations throughout the world within months.) Especially sinister are proteins that pump not just one but many kinds of antibiotics, and thus render bacteria resistant against multiple antibiotics. Curiously, when our own body cells go rogue, proliferate wildly, and evolve rapidly—in cancer—they often use similar efflux pumps to rid themselves of unwanted cancer drugs. These are not only independent solutions to a similar problem but also one of many reasons why the war on cancer is hard to win.
21
The proteins behind these innovations were not created from scratch. They are modified transporters, proteins that are essential in a cell’s daily life, because they ship thousands of molecules—nutrients, waste, building materials—to various destinations within the cell. So should we really call them innovations? The same question arises for the goose’s improved hemoglobin and primates’ color vision. Nature just fiddled with hemoglobin to tighten its binding to oxygen, and it tinkered with opsins to tune their color sensitivity. Neither was a qualitatively new protein. But consider the impact of these changes. Consider the millions of square miles of new habitat opening up to a bird that can traverse
any
mountain range. Consider how much duller our world would be in black and white. And consider the life-and-death change that drug resistance can make to a bacterium. For their dramatic consequences alone, these small changes deserve to be called innovations. They show how minute alterations of no more than a few atoms can have effects that percolate through an organism that is a million times as large and alter the life of its descendants forever.
22