Boost Your Brain (11 page)
Authors: Majid Fotuhi

Forty-three-year-old Christina, a mom of three, was hooked the moment she first clipped on her high-tech pedometer. The tiny step counter, she found, offered the encouragement she desperately needed after eight years in which her primary form of activity was ushering her kids in and out of the car for the drive to soccer or school or kung fu. Suddenly, every step seemed to count. Weighing in at the high end of normal BMI, Christina hoped adding extra steps to her day would help her shed a few pounds. If upping her activity would also help sharpen her thinking and put her on a path to future good health, it would be a bonus well worth the effort.
Christina’s eight thousand or so steps a day began to creep ever upward as she made a greater effort than ever to exercise. Within two weeks of carefully monitoring her activity, she upped the intensity, signing up for a half marathon as an extra motivator. With that, she had three months to train—just the right amount of time to implement a brain fitness program.
Running several times a week would surely help her meet her weight goal. But the question remained: Could moving her body really make a difference in the way her mind moved? There was one way to find out: Christina underwent cognitive testing and an MRI before starting my brain fitness program and then repeated the tests at the close of the three months.
I knew what to expect; even so, I was surprised by the degree of change I saw. In the course of the three months, Christina’s hippocampus had grown a whopping 5 percent. Keep in mind that she was still relatively young and relatively healthy, even before she started the program. Her preprogram lifestyle didn’t expose her excessively to brain shrinkers. To see such dramatic growth in her hippocampus, then, was truly amazing.
And what about her cognitive function? The results of Christina’s paper-and-pencil tests showed more good news: her short-term memory had jumped 15 percent.
It’s nearly impossible to tease out how much of Christina’s improvement was attributed to exercise and how much to other factors, like the DHA supplement she began taking, the regular breathing exercises she added to her day, the ten pounds she lost during the study period, or the intensive cognitive stimulation of helping me write this book. But I believe exercise had a significant impact. Her larger hippocampal size was, most likely, a result of a boost in her BDNF, a spike in the amount of oxygen and in the number of new blood vessels in her brain, and the healthy brain activity she promoted by changing her lifestyle. As you’ll soon read, there’s a great deal of recent research on which to base these assumptions.
Healthy Hippocampus
If you remember my discussion of neurogenesis from
chapter 2
, you’ll recall the research by Fred Gage and others that showed neurogenesis in action in the brains of first animals and then humans. Gage’s research on mice in enriched environments was exciting, but even more exciting was what happened in 1999, when Gage and his team peeled back another layer of the mystery of neurogenesis.
This time, the research team teased out the effects of one particular intervention in the lives of those enriched mice: exercise. Remember, mice who lived in cages with social interaction, toys, and running wheels saw more of their new neurons survive. Exercise alone, however, dramatically increased the number of new neurons being born.
1
Looking at the slides under a microscope, “we were really kind of struck,” Gage recalled for my co-author, Christina, in an interview. “It was actually surprising that running alone could have such a robust effect. You could hold the slide up to the light and see the difference in the brains.”
The next question was, of course, what effect did exercise have on the brains of humans? It’s an area of research that had already been broached from another angle in observational studies. Researchers had long noted that exercise reduced the risk of dementia. But only in recent years have we begun to understand what, exactly, is going on in the brains of those who exercise.
Some of that insight comes from University of Pittsburgh Assistant Professor Kirk Erickson. I often see him at medical conferences and we chat about the incredible malleability of the human brain and how easily it grows. He has studied the topic in depth, and has repeatedly tied exercise to a larger hippocampus and better cognitive performance. In one study, published in 2009, Erickson and his colleagues documented larger hippocampi in older adults who had higher fitness levels.
2
Not only did MRIs reveal the structural changes, but cognitive testing showed those higher volumes also had a real payoff: people who’d exercised more performed better on tests of spatial memory skills.
But was it really exercise that caused the hippocampus to grow? Erickson and his team aimed to find out. To do so, they enlisted 120 cognitively healthy elderly adults aged sixty to eighty who weren’t physically active at the start of the study.
3
Researchers split the participants into two groups, with one group assigned to a regular routine of brisk walking—forty minutes, three times a week—while the other group did stretching and toning exercises. All were supervised by trained personnel, who monitored their heart rates and levels of exertion. And both groups underwent MRIs that measured the size of their hippocampi and blood tests that measured their BDNF levels.
A year later, study participants underwent another round of MRIs to see if their brains showed the effects of their efforts. Those who’d engaged in aerobic exercise saw their hippocampi grow by about 2 percent over the year they’d been exercising. Considering that the hippocampus normally shrinks by 0.5 percent per year as we age, study participants had effectively walked away as much as four years of brain aging. And those who showed greater changes in their BDNF levels saw greater increases in their hippocampal volume—a sign that it was BDNF that was spurring growth in the hippocampus.
By comparison, study participants in the stretching group—who on average didn’t improve their overall fitness—experienced about a 1.4 percent
shrinkage
in their hippocampi over the study period, about what you’d expect for this age group. Those who started out more fit, however, saw less decline—yet another argument for building brain reserve and keeping your hippocampus plump.
How did these changes affect participants’ cognitive performance? It was no surprise to find that the walkers did better in memory-function tests than their stretching peers.
Instead of merely noting a tie between fitness and hippocampal size as his earlier research had, this time Erickson had proof that “an exercise intervention can actually increase the size of brain regions—especially the hippocampus,” he says. In other words, you
can
take an inactive elderly person, make him or her active, and watch that person’s hippocampus grow.
Erickson, in fact, calls it “one of the really important messages” from the study. “Starting an exercise regimen in late life after being physically inactive for most of your life is not futile!” he says. “You can still reap some of the benefits from exercising.”
Most incredible of all, “we know of no medication that can do that,” says Erickson. I agree; this is incredible indeed.
Of course, as reassuring as it is to know that exercise has benefits even in late life, we know, too, that it has clear brain-growing capabilities throughout life.
Children who are fit, for example, have larger hippocampi than their less fit peers, as Erickson and his colleagues demonstrated in a study whose results were published in 2010.
4
For that study, the research team recruited forty-nine nine- and ten-year-olds and measured their oxygen consumption (also known as VO
2
max)—a commonly used measurement of physical fitness—while they ran on a treadmill. All the children then underwent MRI scans.
For Erickson, who’d by then seen the effect of exercise on the brains of the elderly, the results weren’t a given. In late life, when we know the hippocampus is shrinking, “it makes more sense to find an effect where fitness would reduce atrophy,” Erickson explains. “I actually was wondering if we were going to see the same effect in kids, just because kids’ brains are developing at this time.”
But even in young brains—which are growing rather than shrinking—exercise can clearly affect the
rate
of growth. In the children studied, those who were more fit had hippocampi that were 12 percent larger relative to total brain size than their less fit peers.
“The fact that we were able to detect this fitness effect just goes to show you the robustness and the size of fitness and physical activity on the developing brain,” says Erickson. “It’s quite impressive.” Fitter children also did better on memory tests than their less fit peers.
There’s now compelling evidence that exercise has a direct role in increasing BDNF levels too. In 2011, for example, researchers at the University of Dublin linked increased exercise with higher levels of BDNF—and better cognitive performance.
5
For that study, the research team recruited sedentary college students and gave them a face–name matching test. Then, half the students climbed aboard stationary bikes and engaged in a bout of strenuous cycling, while the other half rested. Both groups then took the memory test again and were assessed for levels of BDNF through a blood test.
Participants who engaged in a short period of high-intensity cycling did better on the face-naming task after exercise than they had before it. Those who hadn’t exercised did about the same on the test as they had the first time. The group that had cycled also had higher levels of BDNF in their blood than they’d had before exercising, while the non-cycling group did not.
Five weeks later, with the cycling group continuing to cycle regularly, the team performed the tests again. They found that five weeks of cycling training increased BDNF levels and performance on the face-naming test, leading researchers to conclude that both acute
and
repeated exercise benefit the hippocampus.
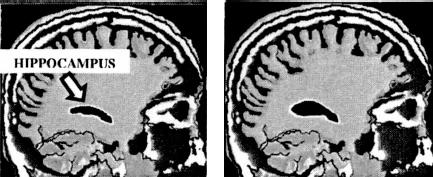
Hippocampal Growth
Before and after images illustrate the type of growth that occurs in the hippocampus with vigorous exercise.
And the Cortex
Having a larger hippocampus, of course, is only part of the story. There’s plenty of evidence that exercise also increases the size of the cortex. One such study comes from Erickson and his colleagues, who examined 299 non-demented elderly people enrolled in the Cardiovascular Health Cognition Study, a longitudinal study that gathered data from 1988 to 2009.
6
Grouping the participants by the number of blocks they reported walking each week, the team studied participants’ initial physical assessments and then followed up with MRI scans nine years later. Four years after that, the team tested the participants for cognitive impairment and dementia.
The results were impressive. Those who’d walked regularly—about six to nine miles a week—had significantly more grey matter in the frontal, occipital, and hippocampal regions than those who walked less. Checking in thirteen years after participants’ initial assessments, Erickson’s team found that those who’d logged six to nine miles a week were far less likely to be cognitively impaired than those who walked the least.
Other research has shown similar changes in grey matter as well as in white matter associated with exercise. In one randomized clinical trial of fifty-nine cognitively healthy people between sixty and seventy-nine years old, for example, researchers at the University of Illinois found that study subjects who engaged in six months of aerobic exercise had more grey and white matter in the frontal and temporal lobes than when they’d started the study. Those who merely did toning and stretching exercises saw no such increase.
7
In 2012, University of California, Los Angeles (UCLA), researchers offered yet more evidence, presenting the findings of their study at the annual meeting of the Radiological Society of North America. Led by Dr. Cyrus Raji, the team examined the lifestyle habits and MRIs of 876 adults aged sixty-nine to ninety-five. Study subjects had varying degrees of cognitive health—some had normal cognitive function, some had mild impairment, and others had Alzheimer’s disease. The team calculated how many calories study subjects burned doing physical activities that ranged from sports, to yard work, to dancing, to cycling. Activity levels varied dramatically: those in the top twenty-fifth percentile burned about 3,400 calories a week, while those in the bottom twenty-fifth percentile burned just 384 calories a week on physical activity.
8
What was going on in the brain was also dramatic: those who’d burned the most calories had 5 percent more grey matter in the frontal, parietal, and temporal lobes—including the hippocampus—than those who burned the least.