Brilliant Blunders: From Darwin to Einstein - Colossal Mistakes by Great Scientists That Changed Our Understanding of Life and the Universe (24 page)
Authors: Mario Livio

This lecture marked the birth of the term “big bang,” which has since been inextricably attached to the initial event from which our universe sprouted. Contrary to popular belief, Hoyle did not use the term in a derogatory manner. Rather, he was simply attempting to create a mental picture for his listeners. Ironically, a scientist who always opposed the idea behind this model coined and popularized the term big bang.
The name has even survived a public referendum. In 1993
Sky & Telescope
magazine solicited suggestions from readers for a better name—an act generally viewed as an attempt at cosmic political correctness. After three judges (including Carl Sagan, the famous astronomer and popularizer of science) sifted through the 13,099 entries, however, they found no worthy replacement. The title of this chapter (“
B
for Big Bang”) was fashioned after the title of a British television science-fiction drama,
A for Andromeda
, written by Hoyle and TV producer John Elliot. The seven-part series aired in 1961, and it featured actress Julie Christie in her first major role.
Fred Hoyle was born on June 24, 1915, in Gilstead, a village near the town of Bingley in West Yorkshire, England. His father was a wool and textiles merchant who was drafted into the Machine Gun Corps and dispatched to France during World War I. His mother studied music, and for a while played the piano in a local cinema, to accompany silent films. Fred Hoyle, who originally planned to be a chemist, studied mathematics at Cambridge, and he demonstrated such talent and accomplishments that he was elected fellow of St. John’s College in Cambridge in 1939. In 1958 he earned the prestigious Chair of Plumian Professor of Astronomy and Experimental Philosophy at Cambridge. Incidentally, George Darwin, Charles Darwin’s son, had held this chair between 1883 and 1912.
Signs of Hoyle’s relish for independence and sometimes dissension were apparent from an early age. He later recalled:
“Between the ages of five and nine, I was almost perpetually at war with the education system . . . As soon as I learned from my mother that there was a place called school that I must attend willy nilly—a place where you were obliged to think about matters prescribed by a ‘teacher,’ not about matters decided by yourself—I was appalled.” His disdain for
convention continued into his university years.
In 1939 he decided to forgo a PhD degree for the “earthy motive,” in his words, of having to pay less income tax!
Not surprisingly, this curiosity-driven independent thinker matured to become a brilliant scientist. In terms of contributions to astrophysics and cosmology, Hoyle was probably the leading figure for at least a quarter century. At the same time, he never shied away from controversy.
“To achieve anything really worthwhile in research,” he once wrote, “it is necessary to go against the opinions of one’s fellows. To do so successfully, not merely becoming a crackpot, requires fine judgment, especially on long-term issues that cannot be settled quickly.” We shall soon discover that Hoyle followed his own advice to a fault.
Even without World War II, 1939 was a critical year for Hoyle. It so happened that one after another, two of his research supervisors left Cambridge for appointments elsewhere. His third advisor was the great physicist Paul Dirac, one of the founders of quantum mechanics—the revolutionary new view of the subatomic microworld. Following the wealth of novel ideas of the 1920s, science of the late 1930s looked dull by comparison. Hoyle later wrote that Dirac told him one day in 1939, “
In 1926 it was possible for people who were not very good to solve important problems, but now people who
are
very good cannot find important problems to solve.” Hoyle took this warning to heart and shifted his focus from pure, theoretical nuclear physics to the stars.
Out of Hoyle’s numerous accomplishments, I want to concentrate here on only a few of his contributions to one particular topic: nuclear astrophysics. Hoyle’s work in this area has become one of the main pillars on which our modern understanding of stars and their evolution rests. Along the way, he solved the puzzle of how the atoms of carbon, the anchor of complexity and life as we know it, formed in the universe. To fully appreciate the significance of Hoyle’s achievement, however, we first need to understand the background against which he produced his masterwork.
Prologue to the History of Matter
On one of the walls of almost every science classroom in the United States, you can find a chart of the periodic table of the elements (figure 19). Just as our language consists of words constructed from the letters of the alphabet, all ordinary matter in the cosmos is composed of these elements. Elements are those substances that cannot be further broken down or modified by simple chemical means.
Dmitry Mendeleyev, a Russian chemist, is generally credited with having noticed (in the mid–nineteenth century) the periodic regularities that are the basis of the periodic table, and with having the foresight to predict the characteristics of elements that had yet to be discovered to complete the table. In many ways, the periodic table is a symbolic representation of the progress achieved since Empedocles’ and Plato’s famous fire, air, water, and earth as the basic constituents of matter. As an amazing aside,
the smallest reproduction of the periodic table was engraved in 2011 onto a human hair belonging to chemist Martyn Poliakoff of the University of Nottingham in the United Kingdom. The engraving was done at the university’s nanotechnology center. (The hair was then returned to Poliakoff as a birthday gift.)
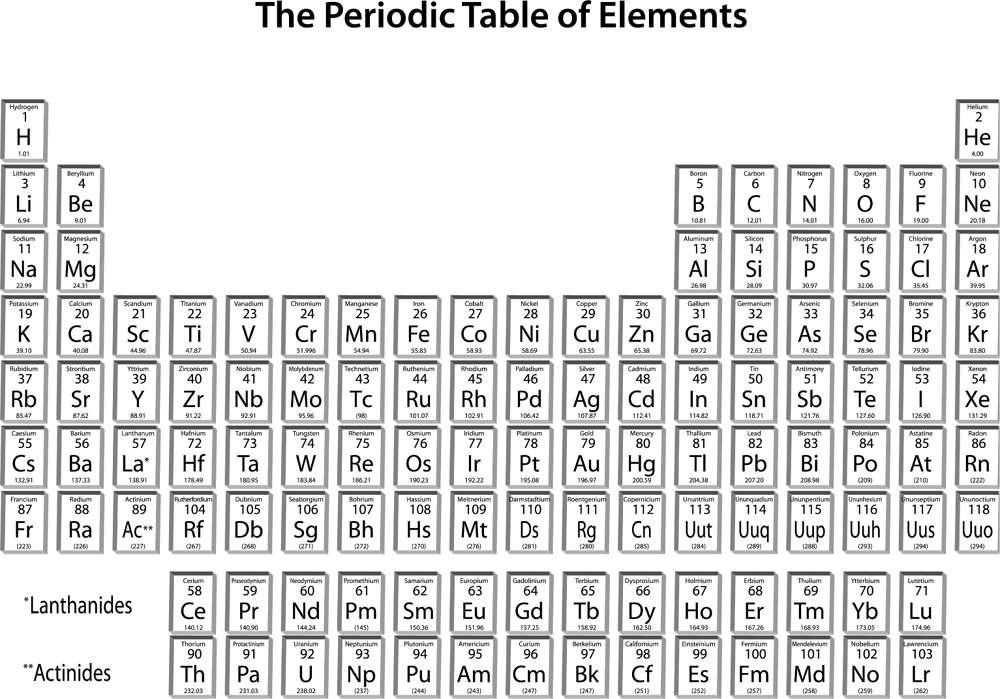
Figure 19
The periodic table currently consists of 118 elements (the latest, ununoctium, was identified in 2002), of which 94 occur naturally on Earth. If you think about it for a moment, this is a fairly large number of primary building blocks, and consequently, it was only a matter of time before someone would ask, Where did all of these chemical elements come from? Or: Could these rather complex entities have simpler origins?
Someone actually did pose these questions even before the publication of the periodic table. In two papers published in 1815 and 1816,
the English chemist William Prout hypothesized that the atoms of all the elements were in fact condensations of different numbers of hydrogen atoms. Astrophysicist Arthur Eddington combined the general idea of Prout’s hypothesis with some experimental results on nuclei by physicist Francis Aston to formulate his own conjecture.
Eddington proposed in 1920 that four hydrogen atoms could somehow combine to form a helium atom. The small difference between the total mass of the four hydrogen atoms and the mass of one helium atom was supposed to be released in the form of energy, through Einstein’s celebrated equivalence between mass and energy,
E
=
mc
2
(“
E
” denotes energy, “
m
” is mass, and “
c
” is the speed of light). Eddington estimated that in this way the Sun could shine for billions of years by converting only a few percent of its mass from hydrogen into helium. Less widely known is the fact that
the French physicist Jean-Baptiste Perrin expressed very similar ideas around the same time. A few years later, Eddington further speculated that stars such as the Sun could provide natural “laboratories” in which nuclear reactions could somehow transform one element into another. When some physicists at the Cavendish Laboratory objected that the Sun’s internal temperature was insufficient to make two protons overcome their mutual electrostatic repulsion,
Eddington is famously said to have advised them to
“go and find a hotter place.” The hypothesis of Eddington and Perrin marked the birth of the idea of stellar
nucleosynthesis
in astrophysics: the notion that at least some elements could be synthesized in the hot interiors of stars. As you might have guessed from the above, Eddington was one of the strongest champions of Einstein’s theory of relativity (especially general relativity).
On one occasion, physicist Ludwik Silberstein approached Eddington and told him that people believed that only three scientists in the entire world understood general relativity, Eddington being one of them. When Eddington didn’t answer for a while, Silberstein encouraged him, “Don’t be so modest,” to which Eddington replied, “On the contrary. I’m just wondering who the third might be.”
Figure 20
shows Eddington with Einstein at Cambridge.

Figure 20
To continue the story of the formation of the elements, we need to remind ourselves of some of the very basic properties of atoms. Here is an extraordinarily brief refresher. All ordinary matter is composed of atoms, and all atoms have at their centers tiny nuclei (the atomic radius is more than 10,000 times the nuclear radius), around which electrons move in orbital clouds. The constituents of the nucleus are protons and neutrons, which are very similar in mass (a neutron is slightly heavier than a proton), each of them being about 1,840 times more massive than an electron. While neutrons bound in stable nuclei are stable, a free neutron is unstable—it decays with a mean lifetime of about fifteen minutes into a proton, an electron, and a virtually invisible, very light, electrically neutral particle called an antineutrino. Neutrons in unstable nuclei can decay in the same fashion.
The simplest and lightest atom that exists is the hydrogen atom. It consists of a nucleus that contains only one proton. A single electron revolves around this proton in orbits the probability for which can be calculated using quantum mechanics. Hydrogen is also the most abundant element in the universe, constituting about 74 percent of all the ordinary (known as
baryonic
) matter. Baryonic matter is the stuff that makes up stars, planets, and human beings. Moving from left to right along rows in the periodic table (figure 19), in each step, the number of protons in the nucleus increases by one, as does the number of orbiting electrons. Since the number of protons is equal to the number of electrons (and they carry opposite electric charges that are equal in magnitude), atoms are electrically neutral in their unperturbed state.
The element following hydrogen in the periodic table is helium, which has two protons in its nucleus. In addition, the helium nucleus also contains two neutrons (which carry no net electric charge). Helium is the second most abundant element, making up about 24 percent of the cosmic ordinary matter. Atoms of the same chemical element have the same number of protons, and this number is called the
atomic number
of that element. Hydrogen has the atomic number 1, helium is 2, iron is 26, uranium is 92. The total number of protons and neutrons in the nucleus is called the
atomic mass
. Hydrogen has the atomic mass of 1; helium, 4; carbon (which has six protons and six neutrons), 12. Nuclei of the same chemical element can have different numbers of neutrons, and those are called
isotopes
of that element. For instance, neon (which has ten protons), can have isotopes with ten, eleven, or twelve neutrons in the nucleus. The common notation for these different isotopes is
20
Ne,
21
Ne, and
22
Ne. Similarly, hydrogen (one proton, or
1
H) also has in nature an isotope usually called deuterium (one proton and one neutron in the nucleus, or
2
H), and an isotope called tritium (one proton and two neutrons, or
3
H).
Returning now to the central problem of the synthesis of the different elements, the physicists of the first half of the twentieth century were faced with a series of questions related to the periodic table. First and foremost: How were all of these elements formed? But also: Why are some elements, such as gold and uranium, extremely rare (hence, their high price!), while others, such as iron or oxygen, are much more common? (Oxygen is about a hundred million times more common than gold.) Or: Why are stars composed mostly of hydrogen and helium?