Life's Ratchet: How Molecular Machines Extract Order from Chaos (16 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

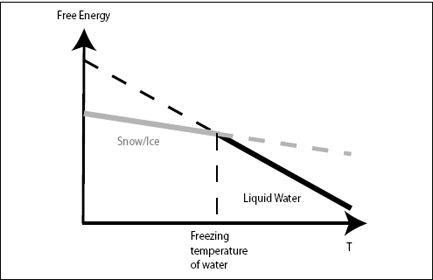
FIGURE 3.1.
Free energy of liquid water (black) and ice or snow (gray) plotted against temperature. Because liquid water has higher entropy, it is represented as a steeper line in the diagram. At low temperatures, ice has lower free energy than liquid water, and water freezes. At high temperature, liquid water has lower free energy (because of the higher entropy), and ice melts.
According to the second law, free energy will eventually be degraded and reach a minimum. If this tendency holds for all natural processes, then the
universe must have started out with an abundance of free energy at the time of the big bang. This is clearly the greatest gift of the universe: Without this gigantic amount of free energy, the universe would be a cold, uniform, and very boring place. How does the universe share its bounty? The big bang started out as pure energy and very little entropy (a single point cannot have much entropy). Shortly after the big bang, energy congealed into energetic particles that whizzed around near the speed of light. The particles collided, annihilated, or appeared out of the expanding fireball, creating chaos where formerly there was simple order. In this expanding fireball, the newborn universe started to create entropy (as well as degrade some of its free energy). As free energy was degraded, new structures were brought into existence—according to Einstein’s equivalence of mass and energy (
E
=
m
c
2
), featureless energy became quarks, electrons, muons, neutrinos, photons, and all their tiny brethren.
After three hundred thousand years, the universe had cooled enough to form the first atoms: Quarks bound together in threesomes called protons and neutrons. Protons and neutrons clustered into nuclei, which captured electrons. Again, the entropy of the universe increased with the release of atomic binding energy (which turned into heat), and something new was created in the process.
The universe continued to cool and became less and less dense. Empty, cold, and no longer able to form nuclei of atomic mass higher than hydrogen and helium, the universe filled to more than 99.9 percent with just these two elements. There is a joke among astronomers that the periodic table really only needs those two entries. All the other elements are so rare, we may as well neglect them (of course, there would be no astronomers if that were truly the case).
Filling the universe with hydrogen and helium was like filling it with fuel. When light nuclei combine in a process called nuclear fusion to form heavier nuclei, they release large amounts of energy (this is what happens in a hydrogen bomb). To make heavier nuclei, two lighter nuclei must collide at very high speed. High speed implies high temperature; as the difficulty of building a nuclear fusion reactor demonstrates, the needed temperatures and densities are difficult to achieve.
This is where gravity lent nature a helping hand. In the early universe, some regions (just by chance) had slightly higher densities than neighboring
regions. These denser regions gravitationally attracted more atoms, gained more mass, and continued to attract more atoms. A once-almost-even distribution of atoms became increasingly clumpy. Atoms clustered together in giant nebulae, separated by giant voids. The nebulae began to collapse under their own weight and, with their continued collapse, became hotter and hotter. Finally, densities within the nebulae became so great and the collisions between nuclei so fast that nuclear fusion began to create heavier nuclei. Hydrogen and helium were cooked into heavier elements, and stars were born.
Stars are the furnaces that “burn” the overabundance of fuel in our universe. Deep within stars, nuclear fusion creates heavier and heavier nuclei, all the way up to iron (even heavier elements are created in large stellar explosions, called supernovae). In fusion, energy is released and streams outward in the form of radiation. Our sun bathes the Earth’s atmosphere in electromagnetic radiation, or light, which is absorbed by molecules and atoms. When molecules absorb this light energy, it is ultimately converted to kinetic energy, making the molecules shake, rotate, or move faster. As these faster molecules collide with slower, less energetic molecules, the faster molecules give up some of their energy in the collision. Soon, this gift of energy from the sun is distributed among many molecules, heating up our atmosphere. The molecular storm, and the abundance of free energy on our planet, come forth from the universe, carried to us by our sun.
This short history of the universe has shown that the degradation of free energy is not all bad. As free energy is dissipated, and the entropy of the universe increased, new structures are born, from quarks to nuclei to atoms to . . . life. The universe continues to share its free energy with abandon. The continual flux of energy is a fact of life—a fact that keeps living systems out of thermodynamic equilibrium. Equilibrium is the state in which all available free energy has been degraded and no usable energy remains. Equilibrium means death. Living beings must avoid equilibrium. As long as we are alive, energy continues to flow through us. In thermodynamics, systems through which energy and matter flow from and to the environment are called
open systems
.
Recognizing that living organisms are open systems is an important step toward our understanding of life—but it is not enough. A small volume of gas within a larger one is also an open system; new molecules enter this volume all the time, while others leave. If left alone, the volume will tend to a state in which, on average, the same amount of molecules enter and leave at any point in time. Moreover, the molecules are not transformed. The molecules that enter are the same as the ones the leave. Consequently, there is no degradation of free energy. Although such a volume is open, it is at equilibrium.
Living systems are different: What enters is not the same as what leaves the system. Living beings gobble up low-entropy energy, degrade the energy, and expel high-entropy energy into the environment. We call such systems
dissipative systems
, because they continuously dissipate free energy into high-entropy energy.
Thus living organisms are
open
,
dissipative
systems. But there is still more. Nature is full of open, dissipative systems that we would not consider alive. A hurricane is an example. It takes a low-entropy source of energy (the large temperature difference between the ocean and the upper atmosphere) and continuously dissipates this energy in a display of awesome power. It dissipates the energy by moving the heat of the ocean to the cool upper atmosphere by convection (air-mass movement due to differences in temperature). The motion of huge masses of air, coupled with forces originating from the Earth’s rotation, soon organize the moving air into a giant rotating storm. As long as there is a supply of warm ocean air, the storm continues to rage, dissipating the heat energy of the ocean as it sweeps across the water. What is most striking about the hurricane is its structure: the rotating swirls of clouds, the eye of the storm in the middle. Many open, dissipative systems show spontaneous emergence of structure, in seeming violation of the second law. But we already know there is no contradiction here. The hurricane increases entropy
overall
far more than it locally decreases it.
The study of far-from-equilibrium systems, and their spontaneously created “dissipative structures,” has led many scientists to speculate that living systems are similarly built. But that would be misleading. A close look at life at the microscopic level shows that it is a tightly controlled dance of sophisticated molecules, designed by evolution. It is not a spontaneous,
wasteful system like a hurricane. Life is a highly efficient process. Efficiency is best achieved when we do not stray too far from equilibrium, because large movements cause friction and, consequently, rapid degradation of low-entropy energy. Life chooses the middle road: By staying away from equilibrium, we stay alive. By staying close to equilibrium, we increase efficiency.
Life is a near-equilibrium, tightly controlled, open, dissipative, complex system. Such a system can only work if its parts are “designed” (by evolution) to push thermodynamics to its limits. Life does not exist despite the second law of thermodynamics; instead, life has evolved to take full advantage of the second law wherever it can. But how can it do this? Life’s engines operate at the nanometer scale, the tiny scale of molecules. But what is so special about this scale that chaos can become structure, and noise can become directed motion?
On a Very Small Scale

There is no excellent beauty that hath not some strangeness in the proportion.
—F
RANCIS
B
ACON
A biological system can be exceedingly small. Many of the cells are very tiny, but they are very active; they manufacture various substances; they walk around; they wiggle; and they do all kinds of marvelous things—all on a very small scale.
—R
ICHARD
F
EYNMAN
, “T
HERE
’
S
P
LENTY OF
R
OOM AT THE
B
OTTOM
,”
LECTURE AT
A
MERICAN
P
HYSICAL
S
OCIETY MEETING
, 1959
I had been impressed by the fact that biological systems were based on molecular machines and that we were learning to design and build these sorts of things.
—K. E
RIC
D
REXLER

I
N MARCH 2011, I ATTENDED THE BIOPHYSICAL SOCIETY meeting to learn what the veterans of this field have been up to and where I should take my own research. Biophysics deals with the physical underpinnings of living systems and the use of physical methods to explore life. When biophysics was founded in the 1800s by researchers such as Helmholtz, it dealt with the parts of living organisms one could easily handle and see. It was a macroscopic science. Today, when you attend the largest meeting of biophysics on the planet (sixty-five hundred participants, over seven hundred posters, every day, for four days), absolutely everything deals with structures only found at the nanoscale. Biophysics today is nanophysics.