Penny le Couteur & Jay Burreson (16 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

The nitro groups are indicated by arrows.
Advances in explosives came about in the nineteenth century when chemists began studying the effects of nitric acid on organic compounds. Only a few years after Friedrich Schönbein destroyed his wife's apron with his experiments, an Italian chemist, Ascanio Sobrero, of Turin, prepared another highly explosive nitro molecule. Sobrero had been studying the effects of nitric acid on other organic compounds. He dripped glycerol, also known as glycerin and readily obtained from animal fat, into a cooled mixture of sulfuric and nitric acids and poured the resulting mixture into water. An oily layer of what is now known as nitroglycerin separated out. Using a procedure that was normal in Sobrero's time but unthinkable today, he tasted the new compound and recorded his comments: “a trace placed on the tongue but not swallowed gives rise to a most pulsating, violent headache, accompanied by great weakness of the limbs.”
Later investigations into the severe headaches suffered by workers in the explosives industry showed that these headaches were due to the dilation of blood vessels caused by handling nitroglycerin. This discovery resulted in the prescription of nitroglyerin for treatment of the heart disease angina pectoris.

For angina sufferers, dilation of previously constricted blood vessels supplying the heart muscle allows an adequate flow of blood and relieves the pain of angina. We now know that in the body nitroglycerin releases the simple molecule nitric oxide (NO), which is responsible for the dilation effect. Research on this aspect of nitric oxide led to the development of the anti-impotence drug Viagra, which also depends on the blood-vessel-dilating effect of nitric oxide.
Other physiological roles of nitric oxide include maintaining blood pressure, acting as a messenger molecule carrying signals between cells, establishing long-term memory, and aiding digestion. Drugs for treating high blood pressure in newborns and for treating shock victims have been developed from these investigations. The 1998 Nobel Prize for medicine was awarded to Robert Furchgott, Louis Ignarro, and Ferid Murad for the discovery of the role played by nitric oxide in the body. Yet in one of chemistry's many ironic twists, Alfred Nobel, whose nitroglycerin-derived fortune would be used to establish the Nobel prizes, personally refused nitroglycerin treatment for the chest pains from his heart disease. He did not believe it would workâonly that it would cause headaches.
Nitroglycerin is a highly unstable molecule, exploding when heated or struck with a hammer. The explosive reaction
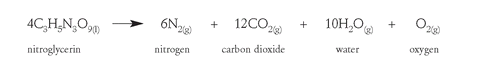 produces clouds of rapidly expanding gases and vast amounts of heat. In contrast to gunpowder, which produces six thousand atmospheres of pressure in thousandths of a second, an equal amount of nitroglycerin produces 270,000 atmospheres of pressure in millionths of a second. Gunpowder is relatively safe to handle, but nitroglycerin is very unpredictable and can spontaneously explode due to shock or heating. A safe and reliable way to handle and set off or “detonate” this explosive was needed.
produces clouds of rapidly expanding gases and vast amounts of heat. In contrast to gunpowder, which produces six thousand atmospheres of pressure in thousandths of a second, an equal amount of nitroglycerin produces 270,000 atmospheres of pressure in millionths of a second. Gunpowder is relatively safe to handle, but nitroglycerin is very unpredictable and can spontaneously explode due to shock or heating. A safe and reliable way to handle and set off or “detonate” this explosive was needed.
NOBEL'S DYNAMITE IDEA
Alfred Bernard Nobel, born in 1833 in Stockholm, had the idea of employingâinstead of a fuse, which just caused nitroglycerin to burn slowlyâan explosion of a very small amount of gunpowder to detonate a larger explosion of nitroglycerin. It was a great idea; it worked, and the concept is still used today in the many controlled explosions that are routine in the mining and construction industries. Having solved the problem of producing a desired explosion, however, Nobel still faced the problem of preventing an undesired explosion.
Nobel's family had a factory that manufactured and sold explosives, which by 1864 had begun to manufacture nitroglycerin for commercial applications such as blasting tunnels and mines. In September of that year one of their laboratories in Stockholm blew up, killing five people, including Alfred Nobel's younger brother, Emil. Though the cause of the accident was never precisely determined, Stockholm officials banned the production of nitroglycerin. Not one to be deterred, Nobel built a new laboratory on pontoons and anchored it in Lake Mälaren, just beyond the Stockholm city limits. The demand for nitroglycerin increased rapidly as its advantages over the much less powerful gunpowder became known. By 1868, Nobel had opened manufacturing plants in eleven countries in Europe and had even expanded to the United States with a company in San Francisco.
Nitroglycerin was often contaminated by the acid used in the manufacturing process and tended to slowly decompose. The gases produced by this decomposition would pop the corks of the zinc cans in which the explosive was packed for shipping. As well, acid in the impure nitroglycerin would corrode the zinc, causing the cans to leak. Packing materials such as sawdust were used to insulate the cans and to absorb any leakages or spills, but such precautions were inadequate and did little to improve safety. Ignorance and misinformation frequently led to terrible accidents. Mishandling was common. In one case, nitroglycerin oil had even been used as a lubricant on the wheels of a cart transporting the explosive, obviously with disastrous results. In 1866 a shipment of nitroglycerin detonated in a Wells Fargo warehouse in San Francisco, killing fourteen people. In the same year a seventeen-thousand-ton steamship, the S.S.
European,
blew up while unloading nitroglycerin on the Atlantic coast of Panama, killing forty-seven people and causing more than a million dollars in damages. Also in 1866 explosions leveled nitroglycerin plants in Germany and Norway. Authorities around the world became concerned. France and Belgium banned nitroglycerin, and similar action was proposed in other countries, despite an increased worldwide demand for the use of the incredibly powerful explosive.
European,
blew up while unloading nitroglycerin on the Atlantic coast of Panama, killing forty-seven people and causing more than a million dollars in damages. Also in 1866 explosions leveled nitroglycerin plants in Germany and Norway. Authorities around the world became concerned. France and Belgium banned nitroglycerin, and similar action was proposed in other countries, despite an increased worldwide demand for the use of the incredibly powerful explosive.
Nobel began to look for ways to stabilize nitroglycerin without losing its power. Solidification seemed an obvious method, so he experimented by mixing the oily liquid nitroglycerin with such neutral solids as sawdust, cement, and powdered charcoal. There has always been speculation as to whether the product we now know as “dynamite” was the result of a systematic investigation, as claimed by Nobel, or was more a fortuitous discovery. Even if the discovery was serendipitous, Nobel was astute enough to recognize that kieselguhr, a natural, fine, siliceous material that was occasionally substituted for sawdust packing material, could soak up spilled liquid nitroglycerin but remain porous. Kieselguhr, also known as diatomaceous earth, is the remains of tiny marine animals and has a number of other uses: as a filter in sugar refineries, as insulation, and as a metal polish. Further testing showed that mixing liquid nitroglycerin with about one-third of its weight of kieselguhr formed a plastic mass with the consistency of putty. The kieselguhr diluted the nitroglycerin; separation of the nitroglycerin particles slowed down the rate of their decomposition. The explosive effect could now be controlled.
Nobel named the nitroglycerin/kieselguhr mixture dynamite, from the Greek
dynamis
or power. Dynamite could be molded into any desired shape or size, was not readily subject to decomposition, and did not explode accidentally. By 1867, Nobel and Company, as the family firm was now called, began shipping dynamite, newly patented as Nobel's Safety Powder. Soon there were Nobel dynamite factories in countries around the world, and the Nobel family fortune was assured.
dynamis
or power. Dynamite could be molded into any desired shape or size, was not readily subject to decomposition, and did not explode accidentally. By 1867, Nobel and Company, as the family firm was now called, began shipping dynamite, newly patented as Nobel's Safety Powder. Soon there were Nobel dynamite factories in countries around the world, and the Nobel family fortune was assured.
That Alfred Nobel, a munitions manufacturer, was also a pacifist may seem a contradiction, but then Nobel's whole life was full of contradictions. As a child he was sickly and was not expected to live to adult-hood, but he outlasted his parents and brothers. He has been described in somewhat paradoxical terms as shy, extremely considerate, obsessed by his work, highly suspicious, lonely, and very charitable. Nobel firmly believed that the invention of a truly terrible weapon might act as a deterrent that could bring lasting peace to the world, a hope that over a century later and with a number of truly terrible weapons now available has still not been realized. He died in 1896, working alone at his desk in his home in San Remo, Italy. His enormously wealthy estate was left to provide yearly prizes for research in chemistry, physics, medicine, literature, and peace. In 1968 the Bank of Sweden, in memory of Alfred Nobel, established a prize in the field of economics. Although now called a Nobel Prize, it was not part of the original endowment.
WAR AND EXPLOSIVESNobel's invention could not be used as a propellant for projectiles, as guns cannot withstand the tremendous explosive force of dynamite. Military leaders still wanted a more powerful explosive than gunpowder, one that did not produce clouds of black smoke, was safe to handle, and allowed for quick loading. From the early 1880s various formulations of nitrocellulose (guncotton), or nitrocellulose mixed with nitroglycerin had been used as “smokeless powder” and are still today the basis of firearm explosives. Cannons and other heavy artillery are not as restricted in the choice of propellant. By World War I, munitions contained mainly picric acid and trinitrotoluene. Picric acid, a bright yellow solid first synthesized in 1771, was used originally as an artificial dye for silk and wool. It is a triple-nitrated phenol molecule and relatively easy to make.
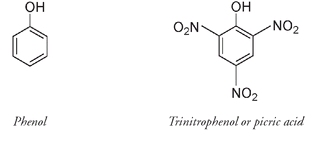
In 1871 it was found that picric acid could be made to explode if a sufficiently powerful detonator was used. It was first employed in shells by the French in 1885, then by the British during the Boer War of 1899-1902. Wet picric acid was difficult to detonate, however, leading to misfiring under rainy or humid conditions. It was also acidic and would react with metals to form shock-sensitive “picrates.” This shock sensitivity caused shells to explode on contact, preventing them from penetrating thick armor plate.
Chemically similar to picric acid, trinitrotoluene, known as TNT from the initials of
tri, nitro,
and
toluene,
was better suited for munitions.
tri, nitro,
and
toluene,
was better suited for munitions.
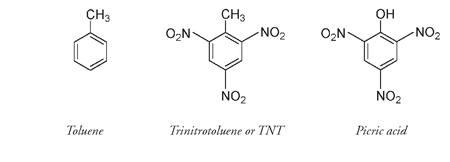
It was not acidic, was not affected by the damp, and had a relatively low melting point so it could be readily melted and poured into bombs and shells. Being harder to detonate than picric acid, it could take a greater impact and thus had better armor-penetrating ability. TNT has a lower ratio of oxygen to carbon than nitroglycerin, so its carbon is not converted completely to carbon dioxide nor its hydrogen to water. The reaction can be represented as
Other books
The Royal Family by William T. Vollmann
Midnight Marriage: A Georgian Historical Romance (Roxton Series) by Brant, Lucinda
Runaway Nun (Misbegotten) by Voghan, Caesar
The Elusive Heiress by Gail Mallin
Billie Holiday by John Szwed
La sombra by John Katzenbach
The Haunting by Garcia, Nicole
Fashion In The Time Of Jane Austen by Sarah Jane Downing
Blood Warrior (The Arcadia Falls Chronicles #4) by Malone Wright, Jennifer
Kendra Kandlestar and the Crack in Kazah by Lee Edward Födi