Regenesis (20 page)
Authors: George M. Church

Antibodies are produced in the bone marrow, where the cells that put them together generate them in random combinations, somewhat analogously to the manner in which the rotating cylinders of a slot machine click to a stop sequentially, producing a different lineup of pictures with each separate run. The business end of the Y-shaped antibody molecule (technically, its antigen-binding site) is composed of two pairs of protein chains, one of which has two parts, the other, three. The atomic structure of each part is encoded in the DNA by a separate gene segment. The chains are put together in such a way that the type of atoms composing one part can be randomly combined with those that compose the other parts so as to generate a stupendously large pool of different antibody types.
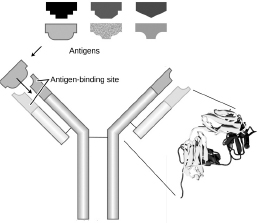
Figure 5.1
How antibodies consisting of four protein chains bind to antigen targets.
The success of an antibody reaction in any given immune response is a function of several factors, including the nature and virulence of the pathogen, the age and healthiness of the person infected, and even, it has been argued, that person's emotional state. In a successful operation, the bound antigens are ultimately ingested by the macrophages and cleared from the body. Viruses that have invaded cells are detected and destroyed by the CD8, or killer T cells, which send them a message telling them to self-destruct, thereby initiating the process of programmed cell death, or apoptosis (pronounced ape-oh-tosis).
The fact that billions of people are alive and well across the planet is testimony to the extraordinary robustness of the immune response over the millennia of our existence. The fact that millions die each year of infectious diseases reminds us that for all its effectiveness, the immune system is nevertheless riddled with defects. Reason enough to bring synthetic genomics into the picture.

When Congress passed a sweeping health care reform bill in 2010, everyone knew that the legislation would end up costing billions if not trillions of dollars. The specifics of the program were largely confined to customary, established, and time-honored (perhaps even hidebound) health care practices. But synthetic genomics will make it possible to go beyond standard practices in at least two ways. One is by providing entirely novel methods of treating existing diseases. Mostly, this involves genomically engineering microorganisms or nonhuman mammalian species for the
purpose of performing specific medical interventions. The other is by reengineering the human genome itself for the purpose of preventing many diseases from occurring in the first place. The second application would be radical and far-reaching in its effects.
Admittedly, the notion of altering our own genes is an idea that takes some getting used to: if certain people are frightened by genetically modified foods, they're going to be stricken with existential terror at the thought of genetically reengineered human beings. But if introducing small, surgical alterations to our genome would make us immune to all viruses, known or unknown, it would revolutionize medicine. Medical treatments for viral diseases would be obsolete. The diseases just wouldn't exist (just as smallpox no longer does), and the cost savingsâboth in dollars and in human miseryâwould be immense. But synthetic genomics could radically improve human health even while leaving the human genome intactâby changing the genomes of other organisms.
One problem in treating cancer is that the chemical agents used in chemotherapy to kill cancer cells often kill healthy cells too. Scientists have proposed various schemes for delivering the chemicals selectively to the cancer tumor alone. For example, physicist Alex Zettl at the University of CaliforniaâBerkeley has suggested placing the chemicals inside tiny, radio-controlled carbon nanotubes that would be guided to the tumor and then triggered to release their anticancer agents. Making this work would require a whole infrastructure of these new devices, and a nontrivial amount of engineering, but it's nevertheless possible.
But there's another way of accomplishing the same goal biologically. In 2006, J. C. Anderson and a group of molecular biologists at various research institutions in California outlined a scheme for engineering
E. coli
bacteria to attack and destroy cancer cells, thereby creating what amounts to a bacterial drug delivery system. In this case, ironically, bacteria would no longer cause disease but cure it.
Anderson and his colleagues advanced their proposal in the
Journal of Molecular Biology
, with “Environmentally Controlled Invasion of Cancer Cells by Engineered Bacteria.” Here they pointed out that after being injected into the bloodstream some bacterial species,
E. coli
among them, are naturally drawn toward tumors, and furthermore are preferentially
attracted toward solid tumors including bladder, brain, and breast cancers. They might do this because of disruptions in the blood flow or some other aspect of the microenvironment near the tumor cells.
To this natural partiality of
E. coli
for cancerous tumors, the researchers added two genetic features from other organisms. First they gave their microbe the ability to invade cells. They did this by taking a gene from a bacterium called
Yersinia pseudotuberculosis
that when expressed by
E. coli
would allow the latter organism to adhere to and then invade mammalian cells. They called this newly engineered, invasive organism
inv+ E. coli
, and experimentally demonstrated that it could enter skin, liver, and even bone cancer cells without difficulty.
Then, to make sure that the new organism would enter mainly cancerous calls, and not healthy cells, they programmed a second discriminating ability into
inv+ E. coli
. Cancer cells have higher densities than normal cells, and so the researchers wanted
inv+ E. coli
to be able to sense the densities of different environments. They gave it a sensor. They took from the marine bacterium
Vibrio fischeri
a genetic circuit called
lux
that enables the organism to distinguish among varying cell densities in its normal aqueous environment. Inserting the
lux
gene into
inv+ E. coli
gave the researchers a genetically rewired bacterium that could distinguish among cells of different densities and selectively invade only higher-density cells. What if the
lux
bacterial circuits mutate? The cells are only used for a brief time and a few molecular mistakes in an ocean of such noise seems acceptable. In any event, this will be extensively tested in clinical trials.
Still, that is only half the story. “Specific invasion of tumor cells is only one component of an anticancer bacterium,” the experimenters said. “Once inside target cells, a cytotoxic or immuno-stimulatory response must instigate destruction of the tumor. Various bacteria have been engineered for this effect including
Salmonella
that metabolize a chemotherapeutic pro-drug at tumor sites.”
Such a biodevice raises several other possibilities, because once you have a bacterium that can sense the microenvironment of a tumor, invade it, and release certain chemicals inside it, you then have a general platform
for a range of similar devices that can also selectively release live vaccines, probiotics, or even genes into predefined target cells.
In the Church lab at Harvard we are working to make these invasive bacteria safer. This is important because bacterial cells can cause inflammatory responses in the host. But we can engineer the bacterial cell wall so that the cell does not elicit this response. Establishing a new counter (of cell divisions or of time) in the synthetic circuitry of the cell will ensure that these invasive cells don't over-replicate in the nutritionally rich environment of the human body. Alternatively, we can limit replication by engineering into the cell a nutritional dependence on something not present in the body (e.g., azido-phenylalanine) and by keeping this nutrient present as the bacteria grow and until they are injected into the patient. Additional safety features include engineering the genomes to be incompatible with DNA in other bacteria so that genetic information cannot flow in from or out to the environment, possibly by changing the core translational code of the cell (as described below).
The cancer-killing stealth bacterium is one scheme for improving human health through genomic engineering. A second is to custom-build antibodies to fight specific diseases. Such antibodies can then be produced in mass quantities and introduced into the bloodstream of patients suffering from those conditions.
One of the most promising advances in medical biotechnology was the development during the 1970s of monoclonal antibodies. Monoclonal antibodies (mAbs) are identical to each other because they are made by immune system cells that are clones of a unique parent cell. Because of their extreme specificity of effect, monoclonal antibodies were thought to be “magic bullets” against various illnesses.
The original idea was to produce mAbs in vitro in a somewhat roundabout process starting from mice that had been injected with a specific antigen, and ending with vials of identical antibodies that would be tailored to neutralizing that antigen. The antibodies would then be injected into human patients, immunizing them to the disease in question.
There was a problem, howeverâthe monoclonal antibodies that had originated within mice were rodent antibodies, not human antibodies. For
that reason, they were rejected by the patient's immune system and rapidly eliminated from the bloodstream. The patient's immune system produced human antimouse antibodies against the mouse-derived monoclonal antibodies, since it regarded them as antigens. The magic bullet, in other words, backfired and failed to clear the body of the disease (but at least did not kill the patient).
But there was a second act to the drama. Scientists had been producing so-called chimeras, or transgenic species, in which the genes from one organism were merged with those of another, since the 1970s. The first transgenic species were bacteria, but biologists soon progressed to inserting foreign genes into the genomes of animals such as mice, sheep, or cattle, for various research, medical, or commercial (or even novelty) purposes. Later, in the early 1990s, other researchers, including Nils Lonberg at the California medical biotech firm Medarex, realized that it would be possible to insert enough human gene sequences into a laboratory mouse genome to create a transgenic, or “humanized,” mouse. (Lonberg and I were grad students together in Walter Gilbert's lab at Harvard.) The theory was that monoclonal antibodies derived from a humanized mouse would not be recognized as foreign by a human recipient's immune system, and therefore would not be rejected by the body.
As it turned out, the second magic bullet scheme worked, and by 2005 more than thirty-three humanized mouse-derived mAb drugs were in various stages of clinical trials, including several developed by Lonberg, whose company, Medarex, had trademarked the name “HuMAb-Mouse” for its proprietary strain of humanized mice. Inasmuch as its mouse genes for creating antibodies had been inactivated and replaced by human antibody genes, the HuMAb-Mouse had acquired the ability to make antibodies that were fully human.
For Nils Lonberg, the payoff came in 2009 when the Food and Drug Administration approved four HuMAb-Mouse drugs for use in humans, for various illnesses including rheumatoid arthritis, autoinflammatory syndrome diseases, psoriasis, and chronic lymphocytic leukemia.
“The four drugs approved in 2009 really represent only the tip of the iceberg for our transgenic mouse platform,” Lonberg said in 2010. “There
are a lot of exciting drugs behind these in clinical development, and we continue to use the platform for drug discovery.”
