Regenesis (4 page)
Authors: George M. Church

The processes that make up the carbon-nitrogen cycle can be thought of as a form of natural selection for favorable reactions and stable elemental forms (atoms and their isotopes). This seems analogous to the mutation and selection of living species, and still later the mutation and selection of synthetic organisms. Today those five (hydrogen, helium, carbon, nitrogen, and oxygen) of the eighty stable elements are the most abundant in the universe. These processes selectively skipped over weakly represented lithium (3), beryllium (4), and boron (5).
A list of such atomic elements (substances that chemically cannot be broken down further) is a prerequisite for understanding the next level of selection complexityâthe combination of those basic atoms into the compounds (molecules) of nature. Antoine Lavoisier wrote the first comprehensive list of the elements in the first modern chemistry text,
Traité élémentaire de chimie
, in 1789. He listed thirty-one in all, together with light and “caloric” (heat), making up a total of thirty-three “simple substances belonging to all the kingdoms of nature, which may be considered the elements of bodies.” As Lavoisier presented them:
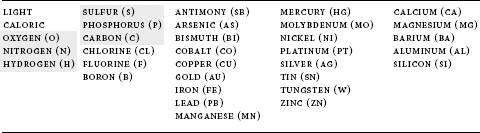
Each element in the table above is followed by the abbreviation that is commonly used in most branches of science, and even within the general cultureâfor example, H
2
O (water), NaCl (salt), and CO
2
(carbon dioxide).
Jöns Jakob Berzelius, who developed an interest in chemistry in medical school, introduced these symbols in 1813. By 1818 he had measured the masses of forty-five of the eighty stable elements. As we will see in
Chapter 3
, as few as six elements may be sufficient to create the major molecules of life: S, C, H, P, O, N (sulfur, carbon, hydrogen, phosphorus, oxygen, and nitrogenâpronounced “spawn”âshaded gray in the table above). These constitute the most abundant elements in living systems; also needed are metal ions such as magnesium (Mg) that are involved in key reactions of these compounds.
These elements chemically combined with one another to form molecules, such as water, as the newly formed earth cooled. How did life arise from nonlife? To understand this, we need to explore the universe of simple, nonliving chemicals. As far as we know, the physical and chemical properties of the elements are set largely by particles in the nucleus (as well as by those in the surrounding electron cloud), and not by the specific arrangement of those particles. For example, it matters only that there are six protons in carbon; the exact structural relationships among the protons are irrelevant. Those six protons, irrespective of how they are arranged in the nucleus, attract and retain an equivalent number of electrons in the surrounding electron cloud.
In molecules, by contrast, the physical arrangement of the component atoms is crucial. For example, a molecule of water, H
2
O, is not just ten protons and ten electrons packed together randomly in a jumble. The order of the atoms and their shape matters. Water is not H-H-O but rather
H-O-H
, meaning that each hydrogen atom can only bind to the oxygen atom, and not to two atoms. Molecules are like intimate social networks. Some atoms, such as hydrogen, tend to make single bonds with only one other atom. Oxygen makes two bonds, nitrogen three, while an atom of carbon can bond with four other atoms. So, water has each hydrogen bonding with one atom, oxygen, and its oxygen bonding with two atoms.
Let's now replace each hydrogen in water with a carbon (keeping each carbon happy with its own three hydrogens): this will give us dimethyl ether, CH
3
-O-CH
3
. So let's check the bonds. The oxygen still has two single
bondsâone to each carbonâand each carbon has four single bonds, three to hydrogens and one to the central oxygen.
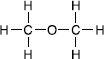
Now we can illustrate the importance of spatial arrangement. If we keep all nine component atoms but rearrange them slightly, say to CH
3
CH
2
OH, we get a radically different set of physical and chemical properties in a molecule called ethanol.
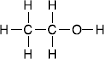
What a difference that simple rearrangement makes! Dimethyl ether boils at -24 degrees C while ethanol boils at +78 degrees C. Many people like to drink ethanol (typically 8 to 15 percent in water), but you would not want to drink dimethyl ether. These rearranged molecules are called isomers of each other (Greek for “the same parts”). Ethanol is an isomer of dimethyl ether: each molecule has two carbons, six hydrogens, and one oxygen, but differently arranged.
Berzelius came up with the concepts and terms for catalysis, polymer, and isomer, among others. He also provided experimental evidence for the law of definite proportions (first stated by the French chemist Joseph Proust), which holds that the proportions of the elements in a compound are always the same, no matter how the compound is made. Even though we have been introducing these ideas by appealing to the simple bonding of discrete atoms, Berzelius discovered them by doing two thousand analyses over the course of a decade, purifying and weighing chemicals and their reaction products. He noticed that the ratios were reproducible and generally came in values that were expressible in whole integers. Berzelius was also the first to recognize the difference between organic compounds
that were derived only from living matter, and all other chemicals, which he lumped together as “inorganic.” This distinction contributed greatly to our understanding of life and set the stage for inquiries into vitalism, the theory that life and its processes are not reducible to the laws of physics and chemistry. Berzelius believed that something kept living matter distinct from nonliving matter. But work done in four areasâthe synthesis of urea, the investigation of mirror molecules, the investigation of polymers (especially of the DNA/RNA polymers), and the self-reproduction of moleculesâargues to the contrary.
Berzelius's protégé Friedrich Wöhler also came to chemistry through the study of medicine. In 1828 Wöhler (accidentally) became the first person to synthesize an organic compound, urea, from an inorganic substance, ammonium cyanate. The reaction in question is NH
3
HNCO â NH
2
CONH
2
. This is a rearrangement of atoms similar to that of the isomers mentioned above. But at the time it was more mysterious, in part because the description of chemicals as precise arrangements of atoms was just becoming evident from experiments. Second, urea was thought to come only from the urine of certain vertebrates as well as, less obviously at the time, other species. Ammonium and cyanate were considered to be inorganic components of minerals.
Wöhler's synthesis of urea was arguably the first great challenge to vitalism
. Since then, scientists have tried to make ever more complex organic living systems from inorganic or otherwise simple nonliving atoms and molecules. With hindsight, urea was a very simple case (consisting of just eight atoms of carbon, hydrogen, oxygen, and nitrogen) and was thus poised for success in this first of five grand challenges to vitalismâall of which reflect milestones in practical synthetic biology as well.
The second challenge to vitalism concerns the phenomenon of the handedness of molecules
â
one of the distinguishing features of living systems. The challenge is to determine whether natural single-handedness can arise spontaneously or be reversed, and if so, what the consequences would be.
The chemistry of life is based on polymers made by linking monomer molecules together in long linear sequences, just as written texts are made
of linear sequences of letters. These two terms share the common root “mer,” from the ancient Greek
meros
for “part.” A monomer, accordingly, is a single molecule (one part), whereas a polymer (many parts) is a molecular structure composed of many similar molecular units bonded together. Amino acids are monomers whereas combinations of them are polypeptides (a.k.a. proteins), which are polymers. The large molecules known as RNA and DNA are also polymersâpolynucleotidesâconsisting of many simple molecular subunits known as nucleotides. Those three types of polymers can bind and catalyze the formation of other polymers as well as the metabolism of the basic components of living things. A single typo in a biopolymer sequence could make the polymer nonfunctional and nonliving. So the
third challenge to vitalism is to find out whether those long, precise sequences could arise spontaneously and possess the functions of life such as catalysis
. Can new kinds of life exist that have no ties to ancient lifeâa truly artificial or synthetic life form?
The fourth challenge is determining whether a fully synthetic chemical network could make a copy of itself and evolve
(i.e., change with time) and in so doing, prolong its own survival. And
the fifth challenge is whether consciousness (or a mind) can arise synthetically
. This will be addressed in the Epilogue.
This section will consider the second challenge to vitalism: biomolecular handedness. There are six compelling reasons to care about handedness.
First, when we inspect meteorites and other matter that has fallen to the earth from space, we look for an excess of molecules of the same handedness (one “enantiomer,” meaning one of a pair of molecules that are mirror images of each other). In space there are more molecules of one specific handedness than of the other. Does this mean that life arose far away and landed here, or rather that one hand is more likely to spontaneously arise or survive? The answer to this question has profound implications for our place in the universe.
Second, the two different hands have different pharmacological effects. The drug thalidomide was used in Europe between 1957 and 1961 to treat morning sickness in pregnant women. Thalidomide was made chemically and not biologically and hence both hands were made in relatively equal amounts. It turns out that one hand cures the morning sickness while the other causes severe limb malformations in the developing fetus (a result described by the BBC as “one of the biggest medical tragedies of modern times”).
Third, chemicals whose molecules exist in only one spatial arrangement tend to be more economically valuable than those that are mixtures of molecules having a given arrangement together with those of their mirror images. The “unnatural” versions are more expensive (1,400-fold more for the amino acid isoleucine).
Fourth, the oceans contain a large mass of carbon trapped in the form of recalcitrant dissolved organic matter (the ominous sounding RDOM), much of which consists of mirror-image forms of easily recycled (nonrecalcitrant) matter. The handedness of these trapped carbon molecules causes them to persist in the oceans for millennia.
Fifth, the ability to reverse the handedness of useful polymers, such as cellulose, wool, and silk, could retard decay. Biodegradable plastics may come to be seen as a mixed blessing. The usual route of biodegradation is through release of carbon dioxide, which is currently an unwelcome output. Also, the energy normally expended in recycling or replacing degraded polymer products might be saved in some cases.