Silent Spring (5 page)
Authors: Rachel Carson

F
O
R
T
H
E
F
I
R
S
T
T
I
M
E
in the history of the world, every human being is now subjected to contact with dangerous chemicals, from the moment of conception until death. In the less than two decades of their use, the synthetic pesticides have been so thoroughly distributed throughout the animate and inanimate world that they occur virtually everywhere. They have been recovered from most of the major river systems and even from streams of groundwater flowing unseen through the earth. Residues of these chemicals linger in soil to which they may have been applied a dozen years before. They have entered and lodged in the bodies of fish, birds, reptiles, and domestic and wild animals so universally that scientists carrying on animal experiments find it almost impossible to locate subjects free from such contamination. They have been found in fish in remote mountain lakes, in earthworms burrowing in soil, in the eggs of birdsâand in man himself. For these chemicals are now stored in the bodies of the vast majority of human beings, regardless of age. They occur in the mother's milk, and probably in the tissues of the unborn child.
All this has come about because of the sudden rise and prodigious growth of an industry for the production of man-made or synthetic chemicals with insecticidal properties. This industry is a child of the Second World War. In the course of developing agents of chemical warfare, some of the chemicals created in the laboratory were found to be lethal to insects. The discovery did not come by chance: insects were widely used to test chemicals as agents of death for man.
The result has been a seemingly endless stream of synthetic insecticides. In being man-madeâby ingenious laboratory manipulation of the molecules, substituting atoms, altering their arrangementâthey differ sharply from the simpler insecticides of prewar days. These were derived from naturally occurring minerals and plant productsâcompounds of arsenic, copper, lead, manganese, zinc, and other minerals, pyrethrum from the dried flowers of chrysanthemums, nicotine sulphate from some of the relatives of tobacco, and rotenone from leguminous plants of the East Indies.
What sets the new synthetic insecticides apart is their enormous biological potency. They have immense power not merely to poison but to enter into the most vital processes of the body and change them in sinister and often deadly ways. Thus, as we shall see, they destroy the very enzymes whose function is to protect the body from harm, they block the oxidation processes from which the body receives its energy, they prevent the normal functioning of various organs, and they may initiate in certain cells the slow and irreversible change that leads to malignancy.
Yet new and more deadly chemicals are added to the list each year and new uses are devised so that contact with these materials has become practically worldwide. The production of synthetic pesticides in the United States soared from 124,- 259,000 pounds in 1947 to 637,666,000 pounds in 1960âmore than a fivefold increase. The wholesale value of these products was well over a quarter of a billion dollars. But in the plans and hopes of the industry this enormous production is only a beginning.
A Who's Who of pesticides is therefore of concern to us all. If we are going to live so intimately with these chemicalsâeating and drinking them, taking them into the very marrow of our bonesâwe had better know something about their nature and their power.
Although the Second World War marked a turning away from inorganic chemicals as pesticides into the wonder world of the carbon molecule, a few of the old materials persist. Chief among these is arsenic, which is still the basic ingredient in a variety of weed and insect killers. Arsenic is a highly toxic mineral occurring widely in association with the ores of various metals, and in very small amounts in volcanoes, in the sea, and in spring water. Its relations to man are varied and historic. Since many of its compounds are tasteless, it has been a favorite agent of homicide from long before the time of the Borgias to the present. Arsenic is present in English chimney soot and along with certain aromatic hydrocarbons is considered responsible for the carcinogenic (or cancer-causing) action of the soot, which was recognized nearly two centuries ago by an English physician. Epidemics of chronic arsenical poisoning involving whole populations over long periods are on record. Arsenic-contaminated environments have also caused sickness and death among horses, cows, goats, pigs, deer, fishes, and bees; despite this record arsenical sprays and dusts are widely used. In the arsenic-sprayed cotton country of southern United States beekeeping as an industry has nearly died out. Farmers using arsenic dusts over long periods have been afflicted with chronic arsenic poisoning; livestock have been poisoned by crop sprays or weed killers containing arsenic. Drifting arsenic dusts from blueberry lands have spread over neighboring farms, contaminating streams, fatally poisoning bees and cows, and causing human illness. "It is scarcely possible ... to handle arsenicals with more utter disregard of the general health than that which has been practiced in our country in recent years," said Dr. W. C. Hueper, of the National Cancer Institute, an authority on environmental cancer. "Anyone who has watched the dusters and sprayers of arsenical insecticides at work must have been impressed by the almost supreme carelessness with which the poisonous substances are dispensed."
Modern insecticides are still more deadly. The vast majority fall into one of two large groups of chemicals. One, represented by DDT, is known as the "chlorinated hydrocarbons." The other group consists of the organic phosphorus insecticides, and is represented by the reasonably familiar malathion and parathion. All have one thing in common. As mentioned above, they are built on a basis of carbon atoms, which are also the indispensable building blocks of the living world, and thus classed as "organic." To understand them, we must see of what they are made, and how, although linked with the basic chemistry of all life, they lend themselves to the modifications which make them agents of death.
The basic element, carbon, is one whose atoms have an almost infinite capacity for uniting with each other in chains and rings and various other configurations, and for becoming linked with atoms of other substances. Indeed, the incredible diversity of living creatures from bacteria to the great blue whale is largely due to this capacity of carbon. The complex protein molecule has the carbon atom as its basis, as have molecules of fat, carbohydrates, enzymes, and vitamins. So, too, have enormous numbers of nonliving things, for carbon is not necessarily a symbol of life.
Some organic compounds are simply combinations of carbon and hydrogen. The simplest of these is methane, or marsh gas, formed in nature by the bacterial decomposition of organic matter under water. Mixed with air in proper proportions, methane becomes the dreaded "fire damp" of coal mines. Its structure is beautifully simple, consisting of one carbon atom to which four hydrogen atoms have become attached:
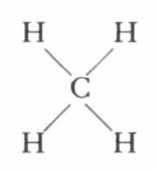
Chemists have discovered that it is possible to detach one or all of the hydrogen atoms and substitute other elements. For example, by substituting one atom of chlorine for one of hydrogen we produce methyl chloride:
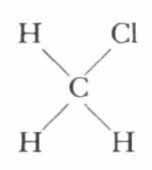
Take away three hydrogen atoms and substitute chlorine and we have the anesthetic chloroform:
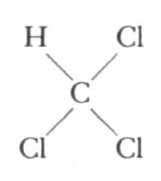
Substitute chlorine atoms for all of the hydrogen atoms and the result is carbon tetrachloride, the familiar cleaning fluid:
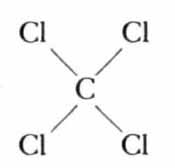
In the simplest possible terms, these changes rung upon the basic molecule of methane illustrate what a chlorinated hydrocarbon is. But this illustration gives little hint of the true complexity of the chemical world of the hydrocarbons, or of the manipulations by which the organic chemist creates his infinitely varied materials. For instead of the simple methane molecule with its single carbon atom, he may work with hydrocarbon molecules consisting of many carbon atoms, arranged in rings or chains, with side chains or branches, holding to themselves with chemical bonds not merely simple atoms of hydrogen or chlorine but also a wide variety of chemical groups. By seemingly slight changes the whole character of the substance is changed; for example, not only what is attached but the place of attachment to the carbon atom is highly important. Such ingenious manipulations have produced a battery of poisons of truly extraordinary power.
DDT (short for dichloro-diphenyl-trichloro-ethane) was first synthesized by a German chemist in 1874, but its properties as an insecticide were not discovered until 1939. Almost immediately DDT was hailed as a means of stamping out insect-borne disease and winning the farmers' war against crop destroyers overnight. The discoverer, Paul Müller of Switzerland, won the Nobel Prize.
DDT is now so universally used that in most minds the product takes on the harmless aspect of the familiar. Perhaps the myth of the harmlessness of DDT rests on the fact that one of its first uses was the wartime dusting of many thousands of soldiers, refugees, and prisoners, to combat lice. It is widely believed that since so many people came into extremely intimate contact with DDT and suffered no immediate ill effects the chemical must certainly be innocent of harm. This understandable misconception arises from the fact thatâunlike other chlorinated hydrocarbonsâDDT
in powder form
is not readily absorbed through the skin. Dissolved in oil, as it usually is, DDT is definitely toxic. If swallowed, it is absorbed slowly through the digestive tract; it may also be absorbed through the lungs. Once it has entered the body it is stored largely in organs rich in fatty substances (because DDT itself is fat-soluble) such as the adrenals, testes, or thyroid. Relatively large amounts are deposited in the liver, kidneys, and the fat of the large, protective mesenteries that enfold the intestines.
This storage of DDT begins with the smallest conceivable intake of the chemical (which is present as residues on most foodstuffs) and continues until quite high levels are reached. The fatty storage depots act as biological magnifiers, so that an intake of as little as 1/10 of 1 part per million in the diet results in storage of about 10 to 15 parts per million, an increase of one hundredfold or more. These terms of reference, so commonplace to the chemist or the pharmacologist, are unfamiliar to most of us. One part in a million sounds like a very small amountâand so it is. But such substances are so potent that a minute quantity can bring about vast changes in the body. In animal experiments, 3 parrs per million has been found to inhibit an essential enzyme in heart muscle; only 5 parts per million has brought about necrosis or disintegration of liver cells; only 2.5 parts per million of the closely related chemicals dieldrin and chlordane did the same.