The Red Market (21 page)
Authors: Scott Carney

If we want to live in a world where human lives are priceless and in some ways equal, then the market cannot be the best decider of which people have the right to other people’s bodies. Inevitably even the best systems of tissue donation break down at some point and let in criminal elements. Even if most of the time it works without people being exploited, the crimes, when they happen, are so extreme that they undermine the benefits of the entire system to society at large.
The current ethos that rules red markets around the world is the assumption that there is an ethical way to build a commercial system of flesh exchange on top of altruistic donations. And yet the short supply of altruism around the world makes the overall system unsustainable. When that supply falters, criminal elements look for illicit ways to increase supply.
One solution to the hypocrisy would be to outlaw all monetary exchange for human tissue and bodies. This would include a ban on paying doctors for their services, tissue supply companies, medical transporters, and everyone else involved in the industry along the way. This, of course, would likely strengthen the black market and drive the industry underground while drastically reducing the supply of legal exchanges.
Alternatively, we could do away with the notion of inherent human equality and accept that the body is a commodity like any other. Embracing the market would presuppose that humans can be treated like widgets and force us to accept the inherent unfairness that some people will always supply flesh, while others will consume it. In this formulation it might be possible to regulate away the worst offenses in tissue harvesting and cut incentives to criminal brokering. And yet, what would we lose as a society by formally creating these two distinct classes of people?
In truth, these solutions are not very attractive. As a society we neither want to accept open trade in human tissue, nor do we want to reduce our access to life-extending treatments. In other words, we want to have our cake and eat it, too.
When philosophers and social scientists get to this point in the debate between a market for human tissue and the ethics of harvesting it, someone always looks for a back door and raises the possibility of a synthetic market. If technology created the ethical conundrum, perhaps it can find a way out of it as well.
“WE ARE ON THE
verge of a breakthrough,” says Savvas Koundouros in his breezy IVF office, confident that new stem cell therapies are just around the corner. There is no reason the revolution couldn’t start here. The island of Cyprus is something of a safe haven for doctors who break the rules on the frontiers of medicine. In 1986 Koundouros’s competition, Krinos Trokoudes, made the
Guinness Book of World Records
for impregnating a forty-six-year-old woman through IVF. In a more controversial case, one Cypriot doctor, Panayiotis Michael Zavos, happily proclaimed his desire to thwart the laws so he could become the first doctor to successfully clone a human. He declared 2002 “the year of human clones” and started pushing for a breakthrough in his lab. He kept the location of his office secret—ostensibly to protect the lives and identities of the offspring—and by 2009 he claimed to reporters at the
Independent
that he had tried implanting eleven cloned embryos into women ready to deliver the children. None of the embryos produced a viable offspring, but he has not indicated that he intends to stop his efforts. After all, it took 277 attempts for UK scientists to clone the sheep “Dolly.” The
Independent
quoted Zavos saying that it was only a matter of time until he, or someone else, clones a human.
OUTSIDE OF THE REALM
of novels like Kazuo Ishiguro’s
Never Let Me Go,
in which human clones are cultivated for their replacement organs, human cloning is not going to stop the ceaseless demand for human bodies. And yet researchers around the world are searching for breakthroughs that promise to create steady supplies of artificial (and depersonalized) human tissue. A success could turn the entire world of red markets on its head.
There would be no reason to run a blood farm or steal a kidney if biologically perfect synthetic tissue and organs could be manufactured at industrial levels. No one would need a bone transplant if an injection of stem cells would grow new bone. In transplant circles people wistfully talk about how the future will be regenerative medicine. Given the complexities of the red market today, ultimately regenerative medicine might be the only sane way to unhinge the current market for human body parts and eliminate flesh-harvesting networks.
The first, and arguably most successful, case of a synthetic destroying the market for human materials occurred in 1985, when the biotech giant Genentech synthesized human growth hormone (HGH) with recombinant mRNA. Before that, injections of HGH had been shown effective in overcoming certain types of dwarfism in young children, and body builders learned that HGH could add mass to their frames and bring their muscles to new heights of definition and strength. Sure it was, and continues to be, illegal to use HGH to gain a competitive advantage, thought that hasn’t stopped athletes from demanding it. But HGH wasn’t easy to come by. Prior to 1985, the only way to get HGH was to harvest the pituitary glands from cadavers and almost literally squeeze out the juices from the tiny organ to extract the hormones. The process was inefficient, required a large number of glands to make a dose, and had no steady source of supply.
From the 1960s to the mid-1980s morticians and pathologists that conducted autopsies for police departments harvested hundreds of thousands of pituitaries and sold them to pharmaceutical companies who processed them into an injectable solution. It was standard practice and most people never knew that their loved ones were being cut up and sold. Still, HGH was so expensive and hard to find that hospitals had to guard their stocks tightly or thieves would steal it from their storerooms to sell on the black market.
When synthetics hit the market, the trade in pituitary glands vanished overnight. While the process to synthesize HGH isn’t easy or particularly cheap, the hormone was suddenly available in much larger quantities than it had been before. It was also available without the specter and negative health side effects of injecting something that was harvested from a cadaver. While doping with HGH continues to plague the sports world, the supply chain has moved on from its roots in the flesh bazaar.
Synthetics offer hope across a spectrum of red markets. Today there are dozens—if not hundreds—of small companies investing in regenerative research that could one day pay off. By and large they fall into two distinct camps: First are laboratories exploring ways to stimulate the body’s own ability to cure itself by either providing cellular raw materials that can cure broken or aging parts, or unlocking hidden genetic codes that will activate dormant curative properties. Researchers in this vein assume that the body already knows how to cure its problems, and just needs a little help to finish the job. This includes the world of stem cell therapies, gene therapies that unlock regenerative potential, and almost the entire field of alternative medicine.
The second school of regenerative medicine is often agnostic on the subject of self-regeneration, but assumes that with enough data, we can use our technical know-how to fix any bodily problem. Replacement bodies can be built from the ground up and surgically manipulated to function. This is the realm of prosthetic and robotic limbs, synthetic tissue and organs, and artificial hormones.
Both schools of thought have made basic advances that have kindled the hope of millions of patients. And yet the research prognosis is so far out that neither is likely to turn off the demand for human tissues any time soon.
For example, take stem cells and any one of the hundreds of anecdotal miracle events that happen, and get reported on, every year.
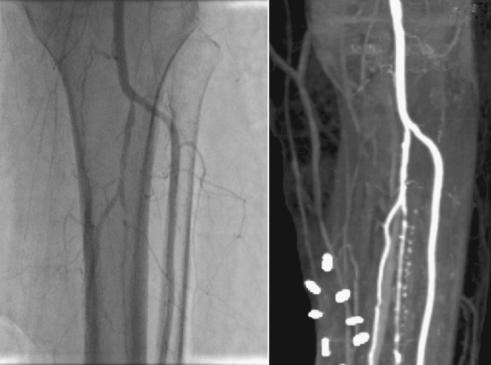
Vamal Cattacha’s angiogram shows veins in her leg after an experimental stem cell treatment in Chennai, India. The image shows that new veins have grown in as bright white streaks. If the treatment had not been successful, doctors would have had to amputate her leg. The success has not been duplicated since.
In 2006 a seventy-year-old diabetes patient named Vamal Cattacha was reclining in a hospital bed on an airy medical ward in Chennai. She smiled when I entered the room with S. R. Subrammaniyan, a doctor who was wearing a blue button-down shirt and a pressed white lab coat. Without his help, Cattacha was sure that she would never walk again, and I had come along to document her recovery. Earlier that year she noticed a small pinprick-sized cut on her leg, but assumed it would go away. After a few weeks of not paying attention, it had spread into a twenty-two-inch-long gaping ulcer that stretched from the heel of her foot to her mid-calf.
Leg ulcers like hers are common among diabetes patients. As the disease progresses, veins and arteries in the limbs start to atrophy and disappear, making it hard to recover from seemingly insignificant injuries. Small wounds can lead to big problems and often leave people permanently disabled. According to the American Diabetes Association, ulcers like Cattacha’s are responsible for almost 60 percent of nontraumatic amputations in American hospitals. That’s roughly eighty-two thousand amputations a year in the United States. While there is no official number of amputations in India, diabetes rates are even higher on the subcontinent than in the United States.
Cattacha, however, wasn’t willing to take amputation for an answer. She traveled across South India looking for a doctor who could give her any other options. Even a sliver of hope would do. Eventually she met Subrammaniyan, who had recently partnered with a Japanese stem cell company that wanted to test a new breed of regenerative therapies. Other than the gaping wound on her leg, Cattacha was in pretty good health, which made her a good candidate for an experiment.
The plan was deceptively simple. Subrammaniyan drew adult stem-cell-rich bone marrow from Cattacha’s hip and then separated the stem cells from the normal blood cells with a centrifuge. Over the next week he injected a solution that he made out of the stem cells into her leg and grafted a piece of skin over the wound.
Within sixty days, the ulcer had visibly healed, and bright white signatures of arteries streaked across her post-treatment angiograms. Before the injections her leg had had almost no circulation at all. The stem cells had apparently re-formed significant lengths of her atrophied circulatory system.
Subrammaniyan called the media, and soon local papers were extolling the virtues of the out-of-the-way medical center. And yet despite the success, the doctor’s explanation was enigmatic. “No one quite knows how it works,” said Subrammaniyan, “but somehow, once injected, the stem cells know how to transform into the right sort of cells.”
For Cattacha the pain was gone, but an isolated success story is not a revolution in stem cell treatment. When I originally reported on the treatment for
Wired
News,
doctors in the United States cautioned against reading too much into the results.
“This was a single case with no controls,” wrote Geoffrey Gurtner, associate professor of surgery at Stanford University and an expert on diabetes care, in an e-mail. “We know that in any disease state, some patients get better even in the absence of care for reasons we do not entirely understand.”
Over the next three years, while I was living a half mile from the hospital, I checked in with the doctors to see if they were ever able to replicate the success, or at least give a more definitive explanation of Cattacha’s recovery. There was never any real news. They continued to test stem cell treatments on humans—occasionally issuing press releases about paralyzed patients who regained partial movement after an injection similar to Cattacha’s. In every other case I checked into, what appeared to be a miracle ended up not being repeatable, the results ambiguous.
The underlying problem is that for the most part no one really understands how stem cells work in a therapeutic setting. The theory is that the body knows how to heal itself and that stem cells somehow know where they are most needed in the body and then set about fixing the problem on their own. For the most part researchers see their own role in therapy as delivery agents.
And yet the appeal of the experiment is obvious. With no reliable therapies, a person who has been injured in a traumatic accident, or is suffering from a broken spine or failing organs, doesn’t have much to lose. Is it better to pursue a path of slim hope with doctors who will experiment on their bodies, or feel helpless and trapped in a world of no good options?
IN NEW DELHI, JUST
three hours north of Chennai by plane, Geeta Shroff is a pioneering doctor in experimental stem cell therapy on humans. She’s not so concerned about understanding the exact mechanisms of stem cells as she is about trying new methods and hoping for results. She’s a doctor of last resort for people who have gone everywhere else. In her lab she enthusiastically injects her own embryonic stem cell brew into a stream of patients from around the world, treating broken spinal cords, progressive neurological diseases, and terminally ill people at a cost of $20,000 to $30,000 per treatment.