Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (1382 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
10.03Mb size Format: txt, pdf, ePub
HIV has evolved into several groups: M, N, O, and P. Group M (“main”) is considered the pandemic strain and comprise most strains of HIV. Group O (“outlier”) represents far fewer strains from Cameroon, Gabon, and Equatorial Guinea. Group N (“non-M/non-O”) and group P are represented by very few isolates and have only been documented in Cameroon. Viruses from group M are subsequently divided into 10 distinct subtypes (A–J). HIV testing was originally developed to detect HIV subtype B, the most common subtype in the United States and Europe. The estimated frequency of non-B subtypes in the United States is approximately 2%. The CDC does not recommend routine testing for HIV-2 in settings other than blood centers.

Normal range:
Negative.
Use
Screening of HIV-1 and/or HIV-2 infection, organ transplant donors, testing individuals who have documented and significant exposure to other infected individuals, and testing exposed high-risk individuals for detection of antibody (e.g., persons with multiple sex partners, persons with a history of other STDs, IV drug users, infants born to infected mothers, allied health care workers, and public service employees who have contact with blood and blood products) (Figure
17-1
)
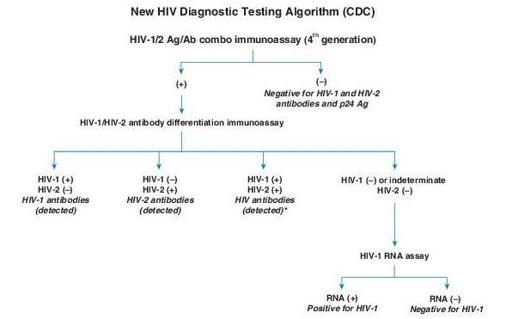
(+) = Reactive (or repeatedly reactive) test result, in accordance with manufacturer’s instructions
(–) = Nonreactive test result, in accordance with manufacturer’s instructions
Italics = Final interpretation; No further testing indicated for the specimen
*
Additional testing required to rule out dual infection with HIV-1/ HIV-2.
Figure 17–1
HIV diagnostic testing algorithm. (From Morbidity & Mortality Weekly Report. June 21, 2013, 62:490–494.)
Interpretation
Positive in HIV infection; a positive screen test is considered “preliminary” and requires confirmation by definitive, specific testing, like HIV-1 RNA or Western blot assay. The patient with a positive test should be told of the result and advised on avoiding risk of transmitting HIV.
A negative screen test is regarded as a true negative, and requires no confirmation; the patient may be informed that the test is negative.
Limitations
Common causes of false-negative results can occur due to acute infection and failure to detect certain HIV subtypes.
Rare causes of false-negative results include immune dysfunction due to defective humoral response or agammaglobulinemia, immunosuppression due to malignancy or medications, delay in seroconversion following early initiation of antiretroviral therapy, and fulminant HIV infection.
False-positive test results for HIV infection have been documented after participation in an HIV vaccine trial.
Other books
The Red Line by R M Reef
Blue Moon by Lisa Kessler
The Blue Herring Mystery by Ellery Queen Jr.
Seaside Reunion by Irene Hannon
UpAndComing by Christi Ann
THE BONDAGE OF LOVE by Yelena Kopylova
Make Me by Charlotte Stein
The Bridal Swap by Karen Kirst
The Private Practice of Michael Shayne by Brett Halliday
Act of Revenge by Robert K. Tanenbaum