Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (997 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
8.32Mb size Format: txt, pdf, ePub
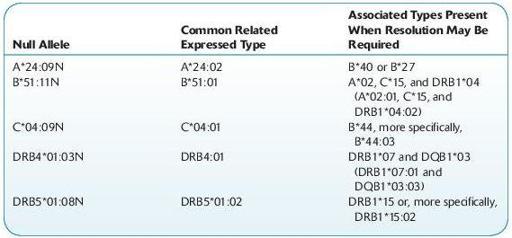
Alleles that have been shown not to be expressed, “null” alleles, have been given the suffix “N.” Based upon regulations required by the American Society of Histocompatibility and Immunogenetics (ASHI) and the National Marrow Donor Program (NMDP), guidelines for null allele typing have been established. The HLA lab is required to test for the following null alleles when alleles and/or haplotypes that have been associated with the specific null alleles exist. Additional CWD null alleles are constantly being updated and incorporated into the required typing list.
Engraftment monitoring (EM) testing
Following HSCT, patients are monitored closely for early engraftment, evidence of graft rejection, or recurrence of the original disease. HSCT creates a donor/recipient chimerism in the patient, which can be quantitatively measured through short tandem repeat (STR) analysis of peripheral whole blood, lineage-specific subsets, whole marrow, or CD34 progenitor cells in the marrow to determine the percent chimerism.
The pretransplant samples used in the EM assay can be the samples used for HLA typing. No additional pretransplant samples from the patient or the donor are necessary. The most common postsamples are, whole blood, T cells (CD3), B cells (CD19/20), myeloid cells (CD15/ CD33/CD66b), NK cells (CD56), whole marrow, and CD34 marrow.
Results are commonly reported as the percentage of donor chimerism within the post-HSCT samples. The assay sensitivity is a key element and has to be considered in results interpretation and reporting, for example, for a postsample with no patient DNA detected and assay sensitivity is 3%, the result should be reported as more than 97% donor DNA, no patient DNA detected, full engraftment.
Suggested Readings
Brand A, Doxiadis IN, Roelen DL. On the role of HLA antibodies in hematopoietic stem cell transplantation.
Tissue Antigens.
2013;81(1):1–11.
Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia.
Blood.
2010;116:2411–2419.
Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: A retrospective study.
Lancet Oncol.
2012;13(4):366–374.
Park M, Seo JJ. Role of HLA in hematopoietic stem cell transplantation.
Bone Marrow Res.
2012;2012:680841.
Pegram HJ, Ritchie DS, Smyth MJ, et al. Alloreactive natural killer cells in hematopoietic stem cell transplantation.
Leuk Res.
2011;35(1):14–21.
Nowak J. Role of HLA in hematopoietic SCT.
Bone Marrow Transplant.
2008;42:S71–S76.
HYDROXYBUTYRATE BETA (BHB)
Definition
In DKA, three ketone bodies are produced: BHB, acetoacetic acid, and acetone. BHB is present in the greatest concentration and accounts for approximately 75% of the three ketone bodies. During periods of ketosis, BHB increases even more than acetoacetate and acetone and has been shown to be a better indicator of ketoacidosis, including subclinical ketosis. Other names for this test include 3-hydroxybutyric acid and ketones. Testing for ketones is generally performed with nitroprusside (Acetest) tablets or reagent sticks. A 4+ reaction with serum diluted 1:1 is strongly suggestive of ketoacidosis. Nitroprusside reacts with acetoacetate and acetone but not with BHB. This is important because BHB is the predominant ketone, particularly in severe DKA. It is, therefore, possible to have a negative serum nitroprusside reaction in the presence of severe ketosis.

Normal range:
0.02–0.27 mmol/L.
Use
Other books
Married In Montana (At The Altar Book 1) by Kirsten Osbourne
Collision by William S. Cohen
Hitman's Desire: A Bad Boy Romance by Riley, Megyn
Twisted by Andrea Kane
Dogs Don't Tell Jokes by Louis Sachar
Wake Up and Dream by Ian R. MacLeod
Pulling Home by Mary Campisi
Loopy by Dan Binchy
Protection for Hire by Camy Tang