A New History of Life (14 page)
Read A New History of Life Online
Authors: Peter Ward

Oil and oxygen when mixed together in the air form an explosive cocktail; a single spark of lightning would cause a reaction to go without stopping. But oil dispersed in water, as little particulates, can only be degraded by the action of microbes. Without efficient recycling, Earth should have experienced a huge imbalance in the carbon cycle. In particular, a large amount of oil should’ve been produced, and an equal amount of oxygen should’ve been pumped into the atmosphere. At this time, we do have evidence for a massive oxidation event at 2.1 GA; it formed one of the world’s largest deposits of pure hematite (Fe
2
O
3
) iron ore—the Sishen mine in South Africa.
15
Earth’s atmosphere must have been supercharged with oxygen at that time, to levels not encountered since, and probably impossible to reach without some deviant biosphere driving it there. If planets orbiting other stars went through the same process, the hyperbaric oxygen in their atmospheres would be waving a spectral flag proclaiming, “We are here, and we solved the photosynthesis problem!”
In fact, the record of carbon isotopes for the period of time between 2.2 and 2.0 billion years ago is so wildly out of balance that geochemists have given it its own name, with the jaw-breaking name
“Lomagundi-Jatuli excursion,” and it is the biggest and longest such event yet found in the entire history of our planet. Most of the carbon being emitted from volcanoes was being sequestered as organic material, releasing oxygen to the air; today this ratio is only about 20 percent. This is the evidence of an Earth with oxygen but without organisms capable of breathing it: wild swings in the carbon cycle caused by cyanobacteria excreted lots of carbon compounds as waste, but with no organisms using these chemicals as food. In fact, the remnants of this sludge appear to exist in the Russian province of Karelia, as a weird rock type called shungite. Today, most of these oil-like compounds would have been quickly biodegraded by microorganisms that breathe oxygen, like the fate of most of the Deepwater Horizon spill. This is direct evidence that the environment choked on hydrocarbons, rather than recycling them directly. As a result oxygen kept rising in content until it was so abundant that it produced an atmosphere supersaturated in oxygen, existing at pressures much higher than today. Had there been any forests, the first spark from lightning would have caused a global forest fire of heat and scope beyond anything that has ever occurred on Earth in the time of forests.
This weird episode in the history of life ended abruptly when evolution produced the first organisms that could
breathe
oxygen efficiently. Special copper-based enzymes evolved to do this, but copper deposits themselves require oxygen-rich environments to form. An entirely new kind of intracellular body came into existence, and it exists still, the organelle called the mitochondria, the major source of energy for eukaryotic cells, which are cells that are larger than their prokaryotic (bacterial) ancestors, as well as being cells that contain walled-off interior “rooms” in the giant (compared to all that came before) cells. The mitochondria has its own little piece of DNA, left over from when it was once a free-living bacterium, a microbe that learned to breathe oxygen efficiently. As a result, it has been enslaved for the past 2 billion years.
It is intriguing to note that the best estimate for the age of the last common ancestor of all eukaryotes is about 1.9 billion years, and that
may mark the time that eukaryotes finally evolved to restore balance to the global carbon cycle. It would seem that the biosphere required over 200 million years of evolution to come up with an adequate response to the presence of the intrinsically poisonous oxygen.
The Long Road to Animals: 2.0–1.0 GA
The time between the great oxygenation event (culminating at ~2.3 GA) and the first appearance of common multicellular life has been called the boring billion. The reason is that (supposedly) virtually nothing happened in terms of major biological change. It is as if the history of life took a snooze. A billion years is a long time for almost nothing to happen. But like so much else, the boring billion has recently been shown to be not so boring. New discoveries are showing us that life was not resting at all. But at the same time, in spite of repeated suggestions to the contrary, there are no animals a billion years in age. Instead, this long interval begins with the first significant oxygen in the atmosphere, and by 2 billion years ago a major revolution in life had occurred—the common occurrence of eukaryotic life, our kind of life, large cells with a nucleus. And while the greatest diversity of these new creatures during this period of time were protozoa, familiar to us as the still-living amoeba, paramecia, euglena, and their cohorts, there appeared some strange larger fossils as well, including one of the most bizarre fossils ever recovered.
The various experts agree that there was probably not enough oxygen between 2.2 and 1.0 billion years ago to support animal life.
1
(This is a good time to quickly summarize the difference between animals, (metazoans) and protozoans. All three are eukaryotes—organisms with large cells that contain a nucleus as well as other smaller organelles, such as mitochondria. Animals and “metazoans” are the same thing. All are composed of more than a single cell during their lives except at fertilization. Protozoans can seem animal-like, in that many are capable of movement and relatively complex behavior. But all of them are composed of only a single cell. Nevertheless, they are far larger and far more complex than bacteria.) Yet if that was agreed on, the reason for this was not. Life was capable of oxygenic photosynthesis, but there should have been far more life than all evidence
suggests. Animals need a good 10 percent of the atmosphere to have oxygen (we are at 21 percent today) and the “photosynthesizers” were not doing their job. The answer, when it finally came, was once again the element that runs through the history of these pages in an ever-repeating pattern: sulfur, usually in the guise of its most toxic and at the same time life-giving form—hydrogen sulfide, molecule of life and death. In a 2009 paper published in the
Proceedings of the National Academy of Sciences
,
2
Harvard paleobiologist Andy Knoll and his colleagues showed that oxygen levels
should
have been higher during the boring billion, but were not. Something was holding them back. The long interval devoid of any kind of real intermediary between the single-celled organisms of the 2.3-billion-year-old great oxidation event and the appearance of larger, multicellular creatures of far, far later in time was real.
There were no life forms that we might call complex in this long interval of time (although we hope it is clear from preceding chapters that even the simplest life forms on Earth are unbelievably complex when viewed at the molecular and chemical scale!). And the reason was an overabundance of single-celled sulfur-using bacteria that were competing with the oxygen-releasing forms. Thus it was that two very different life forms competed for resources coveted by all life—space
and nutrients. The sulfur-requiring microbes, called green and purple sulfur bacteria, are still alive today, but only in the most toxic of places—shallow-water lakes and some seaways that have no oxygen but are shallow enough so that sunlight can penetrate to the levels of the bacteria, allowing photosynthesis. But the problem is that this kind of photosynthesis does not split water apart, and thus does not produce oxygen as a by-product.
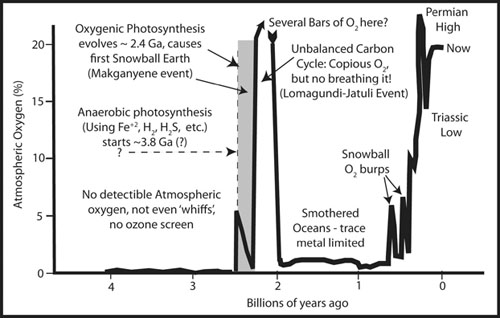
Our new model for the rise of atmospheric oxygen and some of the related events.
Fundamentally, it seems that life was
lazy
. Splitting water is actually a difficult task, which generates all sorts of nasty, toxic compounds. Using H
2
S for photosynthesis instead of H
2
O results in less toxic sulfur compounds, and even many strains of cyanobacteria—if given the choice—will shut down their oxygen-generating machinery and use H
2
S rather than water.
For most of the boring billion the oceans were stratified, with a thin top layer of oxygenated, clean surface water where single-celled green algae took sunshine and used that energy for cell growth, all the while releasing oxygen. But beneath them, perhaps only ten or twenty feet below, was a totally different layer of seawater, and this layer would have extended all the way to the deepest ocean bottom. It would have been purple in color in its uppermost, shallowest regions, stained this color by the untold numbers of the purple sulfur microbes. The water they lived in would have been a fatal poison for most ocean life of our world, as it was filled with toxic hydrogen sulfide all brewed in a near-boiling miasma of liquid brimstone. Even in death they would have helped rob the world of oxygen (unconsciously, of course, although some microbial specialists actually seem convinced that the microbes have always been some kind of sneaky smart). After death, their tiny bodies would have sunk to the bottom, or even stayed in place in water salty or sediment filled enough, and in rotting would have taken even more of the precious few oxygen molecules being produced by the thin layer of oxygen-producing microbes in the surface layer above them. Precious oxygen molecules, destined for the atmosphere and clear oceans, were used up instead in the rotting of a purple demon.
While rare on Earth now, this same stratified system still exists in a few places. One of the most famous is in the Micronesian island of
Palau, in the famous “jellyfish” lakes. Here, large freshwater lakes are filled to bursting with enormous, abundant jellyfish, swimming gracefully through aquamarine and well-oxygenated water. Yet some tens of feet below this crystal lens of clean, oxygen-life-filled water rests a second and deeper stratum, which is dark, and to us creatures of light and oxygen, vile to the extreme. It has little or no oxygen, but is saturated with hydrogen sulfide. And it is dark purple in color, stained by untold numbers of the same purple sulfur bacteria that kept the world unsafe and unavailable for anything needing abundant oxygen for what was to them probably not boring at all.
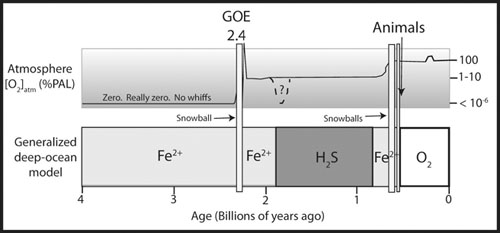
Our revised model for oxygen in the air and sea.
The purple sulfur bacteria and their world needs were finally sent to dank, poisonous back rooms of our world. But they were always there, always ready to take back the world they lost when oxygen finally broke through to higher levels, some 600 million years ago. They can be thought of as the evil empire. And in the Devonian, Permian, Triassic, Jurassic, and middle Cretaceous, this empire struck back, as we will see in subsequent chapters.
Eventually, the balance of sulfur photosynthesizers to oxygen producers changed in oxygen’s favor, possibly triggered by a gradual increase in the area of subaerially exposed continents. Iron eroding from the continents and washing down into the ocean would react rapidly with the sulfur, precipitating it into a heavy, sinking solid mass
of pyrite, keeping it out of the system. This loss would have starved the sulfur bacteria of the one element they could not do without. In addition, continental weathering and erosion generates clay minerals, which bind strongly to organic molecules and bury them in the sediments. If an atom of organic carbon is buried before something can eat it, the molecule of oxygen that was produced when it formed hangs around in the environment, raising the oxygen level and destroying H
2
S. Prompted by two snowball Earth events, each of which seemed to jack up oxygen levels by the postsnowball algal bloom, the environment reached a tipping point of some kind. After the last event, 635 million years ago, the first traces of big animals appeared. It did not take so long to evolve them after all, once hell on Earth was banished.
Most life during the now not-so-boring billion was composed of the long-running champions of life on Earth, the longest-running show of all—stromatolites. Microbes still held sway, just as they had from their first appearance on Earth. But appearing about 2.2 billion years ago a strange new kind of life form appeared. It looks like a thin black spiral, but is certainly not microscopic. Its name is
Grypania
, and its appearance demonstrates that life had made an important advance: the ability to live as “colonies” of cells, held together and bound by membranes. These were the first multicellular organisms.