Alien Universe (24 page)
Authors: Don Lincoln

The first is essentially mandatory. Energy doesn’t drive change, rather energy differences are the source of change. “Thermodynamic disequilibrium” simply means that there are places of higher energy and lower energy. This difference sets up an energy flow, which organisms can exploit for their needs. It’s not fundamentally different from how a hydroelectric power plant works: there is a place where the water is deep (high energy) and a place where the water is shallow (low energy). Just as the flow of water from one side of the dam to the other can turn a turbine to create electricity or a mill to grind grain, an organism will exploit an energy difference to make those changes it needs to survive.
The second requirement is essentially nothing more than saying that life is made of atoms, bound together into more complex molecules. These molecules must be bound together tightly enough to be stable. If the molecules are constantly falling apart, it is hard to imagine this resulting in a sustainable life-form. It is this requirement that sets some constraints on which atoms play an important role in the makeup of any life. Hopefully after this discussion, you’ll understand the reason for the oft-repeated phrase in science fiction “carbon-based life-form.”
Requirement number three is less crucial; however it’s hard to imagine life evolving in an environment that isn’t liquid. Atoms do not move easily in a solid environment and a gaseous environment involves much lower densities and can carry a far smaller amount of the atoms needed for building blocks and nutrition. Liquids can both dissolve substances and move them around easily.
Finally, the fourth requirement might not be necessary for alien life, but it is crucial for Aliens. Certainly multicellular life or the equivalent will not be the first form of life that develops. The first form of life that develops will be of a form analogous to Earth’s single-celled organisms (actually, most likely simpler … after all, modern single cell organisms are already quite complex). In order to form species with increasing complexity, small changes in
the organism will be necessary. Darwinian evolution is the process whereby a creature is created with differences from its parents. The first thing that is necessary is that the organism survives the change. After all, if the change kills it, it’s the end of the road for that individual. Once there are changes that both allow the daughter organism to survive and possibly confer different properties, selection processes become important. Creatures who subsequently reproduce more effectively will gradually grow in population until they dominate their ecological niche.
So let’s talk about these ideas in a little more detail.
Thermodynamic Disequilibrium
The most important consideration for any form of life is the need for thermodynamic disequilibrium. This mouthful of an idea is simultaneously intuitive and counterintuitive.
If you tell someone that energy is necessary for life, you likely won’t get any argument. Plants absorb sunlight, people eat food; the need for energy is self-evident. Yet the reality is a little more subtle. Energy has a technical meaning in science. Energy can be found in a thrown ball, a coiled spring, and a stick of dynamite.
However what life needs is not energy but rather an energy difference. If the energy is the same everywhere, this is not useful. What’s useful are energy differences. To illustrate this subtle difference, consider a water reservoir held back by a dam (
figure 6.1
).
On the water side, everything is equal. While the pressure changes with depth, the uniformity keeps the water from moving around. It tends to stay put. However the water has a kind of energy that scientists call “potential energy.” (Potential energy is the kind of energy where something would move if we let it, like how the water would move if we broke the dam or how an arrow would fly from a stretched bow if the string were released.)
Now imagine that there is a hole in the bottom of the dam. Water would rush out from the water side to the air side. This is in fact how hydroelectric power stations work. The moving water turns a turbine, which generates electrical power.
The crucial takeaway point here is that an energy difference (and a subsequent flow from high energy to lower energy) is central to the creation of electrical power and that this is true in a more general sense. This is what is meant when we say “thermodynamic disequilibrium.” Thermodynamic means energy and disequilibrium means “not equal” or different.
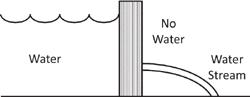
FIGURE 6.1
.
Water being held back by a dam is an example of an energy difference, and this energy difference can be converted into high-pressure water flow that can turn an electrical turbine. Although the energy differences of biology and biochemistry stem from concentrations of chemicals being held back by a cellular membrane, or in the interatomic bonds within molecules, the principle is the same.
Life works the same way. Energy differences allow energy to flow and make the kinds of changes that permit life to exist. For life, it is important to be able to store these energy differences for use when convenient. That way, an organism can move around, carrying its energy source with it. This provides protection against random occurrences that might restrict access to energy.
To get a sense of why this is important, consider a hypothetical alien cow that has to constantly eat to survive. If the cow exists on an ever-growing and ever-present patch of alien grass, there is no problem. However imagine a drought. With the death of the grass, the cow would immediately die, unable to move to a fresh patch of grass. Or imagine a plant that uses sunlight like Earth ones do but that can’t store energy. It would live during the day, but die each night. Without a guaranteed, never-ending energy source, life of these forms is very vulnerable. Energy storage is necessary for life to exist.
It seems likely that life made of atoms (as we are) must exploit energy storage in molecules. Certain atoms can be combined together using available energy (as plants do with sunlight). Later, the energy can be extracted by converting molecules containing a lot of energy into lower energy ones and using the extra energy to live. We do this when we eat a cookie and metabolize sugars or fats. Perhaps an even more intuitive example of this would be when we burn wood. Cellulose combines with oxygen through a series of chemical reactions, resulting in carbon dioxide and water. We know that a fire releases heat—that’s typically the point of fire after all—but what isn’t so obvious is that what we’re seeing when we toast our marshmallows is the transformation of molecules with lots of energy stored in their bonds into ones with less energy.
The Constraints Imposed by Atoms
Scientists know a lot about chemistry, how atoms interact and the properties of the matter they form. Surely this knowledge can tell us a lot about what elements are crucial for life. We are “carbon-based life-forms,” as they say in science fiction. But science fiction also talks about other possibilities. The Horta in the
Star Trek
episode “The Devil in the Dark” was a life-form built around the silicon atom. Larry Nivens’s Outsiders from his
Known Space
series have a biochemistry that includes liquid helium. Given the imagination of science fiction writers, both professional and amateur, I could imagine that sitting in somebody’s drawer is a story about mankind’s encounter with an intelligent race, with platinum bones and molten gold blood, who excreted diamonds. (If someone steals that idea and writes a story, I want a cut of the royalties.) So what does science tell us about the range of atomic combinations that is physically possible? For that, we need to think about some simple molecular requirements of life.
Life cannot exist without atoms combining together to make more complex molecules. Thus the way in which these atoms interconnect is a crucial consideration. While it may be obvious that the rules of chemistry are a defining aspect of any form of life, that statement is pretty vague. We can actually do better than that and discuss in the following text some detailed considerations.
For instance, alien life (and especially Alien life) will require a complex chemistry. Chemicals that perform analogous tasks to our familiar carbohydrates, proteins, DNA, and so on, will have to form molecules consisting of many, interlinking atoms. So two important considerations in the chemistry of life will be to identify atoms that (1) can make many connections to neighboring atoms and (2) can make strong enough connections so that the molecules are stable.
Students of chemistry have long been required to learn about valences, which is essentially the number of bonds the atom of any particular element can make. In order to make complex molecules, an atom will have to be able to connect to many nearby atoms. This can be made incredibly clear by considering the noble gas elements (helium, neon, argon, etc.), which inhabit the rightmost column in
figure 6.2
. These elements do not interact with other atoms. Each atom of the noble elements stands alone. They just don’t participate in chemistry at all. Consequently, we can be certain that these elements do not play a substantial role in any life-form’s metabolism and certainly do not have a structural role in any form of life.
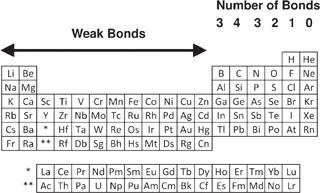
FIGURE 6.2
.
The atoms that make up matter each have a personality, with varying abilities to make stronger and weaker bonds and even different numbers of bonds. This variation between the elements is central to understanding all of matter, including life itself. Chemistry students will find the location of hydrogen (H) to be a little strange, being used to seeing it head the column that includes lithium (Li) and sodium (Na). However, each hydrogen atom can be seen as able to donate or accept an electron to form a bond, thus it could naturally be put in either location.
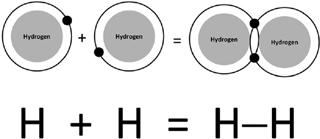
We can then consider the column immediately to the left of the noble elements. This column—which includes hydrogen, fluorine, and chlorine— consists of atoms that can form one bond with a neighboring atom. Since all of these elements act similarly, we can illustrate the point considering just hydrogen. It’s kind of like a room full of one-armed people. They can hold hands with only one other person at a time. In a world in which hydrogen is a building block of life, you can only make very simple molecules, specifically ones consisting of identically two atoms. If hydrogen can form only one bond, then one atom of hydrogen bonds to a second atom. Both atoms form a single bond and the result is a two-atom molecule, as shown in
figure 6.3
. This is true for all elements in that column.
Moving one column to the left, we encounter the two-bond elements. The lightest example of these atoms is oxygen. Since oxygen can form two bonds, it can take on two hydrogen atoms. This is how water is formed, with an oxygen and two hydrogen atoms. Invoking our example of arms, oxygen is a two-armed element. It can hold hands with two hydrogen atoms or hold two hands with another oxygen atom. Moving again one column to the left, we encounter the three-bond elements. In a similar way, a nitrogen atom can connect with three hydrogen atoms and make ammonia.