Life on a Young Planet (18 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

In summary, all biogeochemical signs point, as it were, to Rome. Around 2.4–2.2 billion years ago, it looks like the atmosphere changed. Hiroshi Ohmoto and his colleagues may have a point that oxygen began to accumulate earlier, perhaps locally and certainly in only trace abundances. But it was early in the Proterozoic Eon that the oxygenation of air and water assumed global environmental and biological importance.
Preston Cloud, Dick Holland, and other champions of early Proterozoic environmental transition were right. But why? What factors might push a planet from one long-lived environmental state in which oxygen was rare to another, where oxygen was relatively abundant? A simple answer might be that the evolution of cyanobacterial photosynthesis fomented early Proterozoic oxygen revolution. After all, photosynthesis is the principal source of O
2
on our planet. But the fossil record tells us that cyanobacteria began to diversify at least 300–500 million years before the atmosphere changed, and possibly much earlier.
To understand why photosynthesis alone could not sustain atmospheric transformation, you need only reflect that as you read these pages you are using atmospheric oxygen to respire organic matter, generating carbon dioxide and water in the process. Oxygenic photosynthesis and aerobic respiration form a tight couple in which the products of one metabolism provide the raw materials for the other. In a world where photosynthetic oxygen production is matched by respiratory oxygen consumption, O
2
cannot to build up in the atmosphere and oceans, regardless of how much photosynthesis takes place.
We need to envision processes that can break this coupling in ways that allow oxygen to accumulate. One possibility is to isolate organic matter from reaction with oxygen by burying it in sediments. This prospect changes the picture dramatically. What we previously thought of as a set of
biological
processes acquires a decidedly
geological
cast,
because, globally, organic carbon burial is regulated by the dynamics of sedimentary basins and the deposits that fill them. Alternatively, we can decrease the rate at which oxygen is consumed by continental weathering and reaction with gases supplied by volcanoes. This, as well, inserts geology into Earth’s carbon and oxygen cycles.
In practice, the photosynthesis/respiration couple is always a bit leaky, allowing a small fraction of the organic matter produced by photosynthesis to accumulate in sediments. Balancing this leakage, oxygen is always reacting with continental rocks and volcanic gases (often with the help of bacteria). To alter the face of the Earth, we need to look for
big
events.
Pres Cloud believed that iron in the Archean ocean sopped up the oxygen produced by early cyanobacteria, precluding O
2
buildup in the atmosphere. In his view, increasing photosynthesis by early Proterozoic cyanobacteria swept dissolved iron out of the deep oceans, releasing the brake on oxygen growth. This idea would be attractive, but for one untidy fact, already introduced. Iron formations didn’t disappear 2.2–2.4 billion years ago, when other geologic indicators signal a rise in oxygen levels. The iron-rich rocks of Gunflint formed only 1.9 billion years ago, and a few other iron formations are still younger. This means that rusting oceans couldn’t have released the oxygen brake. It also tells us that the O
2
generated 2.2–2.4 billion years ago was not sufficient to spread oxygen throughout the deep ocean.
Recently, David Catling, Kevin Zahnle, and Christopher McKay, all at NASA’s Ames Research Center, have looked both above and below for explanations of early environmental evolution. They argue that on the late Archean and earliest Proterozoic Earth, some of the methane produced by all those methanogenic archaeans would have reached the upper atmosphere. There, ultraviolet radiation destroyed it, generating hydrogen gas in the process. Unlike most other gases, hydrogen is so light that it can break free of gravity and escape into space. Hydrogen loss would have made it easier for oxygen to gain a foothold at the Earth’s surface. At the same time, rates of volcanism may have declined as the Earth’s interior cooled, reducing the supply of oxygen-consuming gases to the atmosphere. Under these conditions, Catling and colleagues propose, oxygen began to accumulate in the atmosphere and surface ocean, building up until some other brake was applied.
As yet there is no consensus on the particular events that tipped Earth’s environmental scale in planetary middle age. Whole-Earth models like that of Catling and colleagues are attractive, particularly because they are irreversible—once Earth went down the path of hydrogen escape, it could never recapture its oxygen-poor past. There is, however, one more line of evidence that requires consideration. And a third set of Jacob Marley facts.
Until now, our discussions of carbon isotopes have focused on the differences between carbonate rocks and organic matter. A much different type of information is encoded in the
absolute
values of
13
C/
12
C in these materials. As shown in
figure 6.6
, the higher the
13
C/
12
C values of carbonates and organic carbon, the higher the rate of organic matter burial (relative to carbonate deposition) at the time the sediments formed. Carbon isotopic values of early Proterozoic limestones and dolomites are the highest ever recorded globally, supporting the hypothesis that geological changes contributed to the oxygen revolution by promoting the burial of organic matter in sediments.
David Des Marais, also at NASA Ames, has calculated that the amount of oxygen generated by excess organic carbon burial 2.4 to 2.2 billion years ago would have been enough to generate present-day oxygen levels ten times over. The persistence of iron formations until 1.85 billion years ago, however, shows that it didn’t do so. Where did all the oxygen go? Most of it combined with sulfur to form sulfate, giving the sea its modern tang.
Don Canfield was the first to point out an important consequence of this change. We noted earlier that as sulfate levels rose in the ocean, sulfate-reducing bacteria increased concomitantly in importance. Hydrogen sulfide is the by-product of sulfate reduction, so as populations of sulfate-reducing bacteria swelled, more and more H
2
S would have been produced in the deep sea. H
2
S reacts readily with dissolved iron to form pyrite, providing an alternative explanation for the loss of iron formations. Hydrogen sulfide, and not oxygen, might have swept iron from the sea, leaving the deep ocean as anoxic as it was when Warrawoona was young.
How did biology respond to the oxygen revolution? We read, provocatively, of an “oxygen holocaust” in which untold lineages of anaerobic
microorganisms perished. But anoxic environments didn’t disappear 2.2 billion years ago; they simply retreated beneath an oxygenated veneer of surface sediments and water. Indeed, rather than considering the early Proterozoic as a time of environmental
transition
, it may be more profitable to think of it as an interval of environmental expansion—one that that enabled the Earth to support an unprecedented diversity of life. Anaerobic microorganisms retained their critical roles in ecosystem function, roles that they retain today. But organisms that use or at least tolerate oxygen expanded greatly. Aerobic respiration became a dominant metabolism among bacteria, and chemosynthetic bacteria that gain energy by reacting oxygen with hydrogen or metal ions diversified along the interface between oxygen-rich and oxygen-depleted environments.
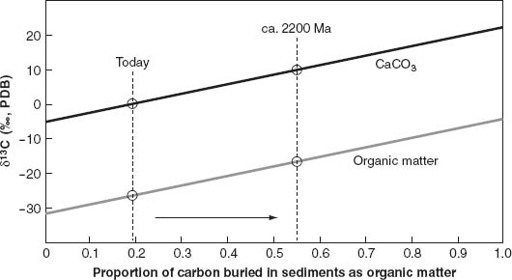
Figure 6.6.
The relationship between carbon burial in sediments and the isotopic composition of carbonates and organic matter, after a diagram by John Hayes. Carbon entering the Earth surface system from the mantle (by way of volcanoes) has a δ
13
C value of about −6‰. (The “delta” notation used by geochemists indicates the difference between
13
C/
12
C in the sample and that of a laboratory standard, expressed in parts per thousand—symbolized by ‰.) If all carbon entering the system were deposited as carbonate, the δ
13
C value of that carbonate would also be −6‰, because, in terms of isotopes, what comes out must equal what went in. For the same reason, if all carbon were buried as organic matter,
its
δ
13
C value would be −6‰. In the real world, where carbon enters sediments as a mixture of carbonate and organic matter, the total isotopic composition of carbon leaving the system must still match that coming in; this is achieved when isotopic compositions for carbonate and organic matter follow the diagonal trend lines shown in the graph. Today, for example, carbon burial in sediments is about 81% carbonate and 19% organic matter, and δ
13
C values of carbonate and organic matter are about 0‰ and −28‰, respectively. In 2.2-billion-year-old rocks, however, carbonate δ
13
C values are commonly about +8‰, whereas the δ
13
C values of organic matter hover around −20‰, suggesting that during this interval, rates of organic carbon burial matched those of carbonate deposition.
In Gunflint time, halfway through our planet’s history, the Earth remained an unfamiliar place. But the trajectory of subsequent evolution had been set. From this time onward, organisms that use or produce oxygen would dominate biology. Indeed, at the Earth’s surface,
only
oxygen and carbon dioxide would ever again be abundant enough to supply the needs of cells larger than a few microns, and oxygen would eventually achieve concentrations able to support large, multicellular organisms. From now on, the Earth would start to become
our
world.
__________
1
The one group of bacteria known to synthesize cholesterol appears to do so using genes gained by lateral transfer from eukaryotes.
7 | The Cyanobacteria, Life’s Microbial Heroes |
If oxygen wrought revolutionary change, cyanobacteria were the heroes of the revolution. Exceptionally preserved fossils in 1.5-billion-year-old cherts from Siberia show that blue-greens diversified early and continue today in little-altered form. The capacity to change rapidly but persist indefinitely may epitomize bacterial evolution.
T
HE ASCENT UP
the Great Wall is tiring. It is cold and it’s damp, there are precious few places to rest, and the footfalls can be slippery. Thank goodness there are no other visitors.
No other visitors? Veterans of Beijing tourist itinerary A (Great Wall in the morning and Summer Palace in the afternoon, with factory tours discontinued) may startle at the thought, but like my North Pole, this isn’t the Great Wall of common experience. My Great Wall is an aptly named sliver of dolomite miles long and hundreds of feet high that separates two pristine rivers in northern Siberia (
figure 7.1
). On the north flank, a familiar stream carries its load of silt and snowmelt westward toward the Arctic Ocean. It is the Kotuikan, the same watery ribbon that carved the Cambrian cliffs encountered in
chapter 1
. The dolomites have also been introduced before. They’re part of the thick sedimentary pile glimpsed downstream beneath the Kotuikan’s Proterozoic-Cambrian boundary succession. In the Great Wall, these older rocks are breathtakingly well exposed—a slender mesa of carbonate beds that lie as flat today as they did when they formed nearly 1.5 billion years ago.
