Life on a Young Planet (26 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Figure 9.3.
Eggs and embryos of early animals, preserved in Doushantuo phosphate. Each fossil is 400–500 microns in diameter. (Images courtesy of Shuhai Xiao)
Later growth stages have not yet been uncovered, so we don’t know what kind of adults might have developed from the Doushantuo embryos. Among living animals, arthropods and related invertebrates most closely approximate the egg case and cell cleavage patterns displayed by these fossils, but this doesn’t mean that recognizable arthropods plied the Doushantuo seaway. In parallel with Doushantuo shales, Guizhou phosphates contain possible sponges and small tubes likely made by simple coral-like organisms (
plate 5c
), but they display no evidence of arthropods or any of the other anatomically complex animals found in phosphatized Cambrian rocks. Around 590–600 million years
ago, then, animal evolution may have begun, but the age of animals was still to come. Doushantuo fossils preserve the smoldering fuse of an evolutionary explosion about to begin.
The Doushantuo Formation is a paleontological wonder, our closest approximation yet to a Precambrian Burgess Shale. Zhang Yun, the quiet pioneer who gave us so many of these remains, died in 1999, but his students continue to plumb Doushantuo rocks for new fossils and further insights into early evolution. The prospect that this paleobiological mother lode will be exhausted in my lifetime is remote.
Thinking back on the fossils found in Great Wall cherts and shales, it becomes clear that biology changed radically between 1.5 billion years ago, when the Siberian rocks were deposited, and 590–600 million years ago, when Doushantuo sediments formed. And further remarkable events were imminent, even as Doushantuo rocks accumulated in South China. In the following chapters, we will explore what came next. But, for now, the task is to fill the evolutionary gap between the Great Wall and Doushantuo biotas.
Multicellular red and green algae are common in Doushantuo assemblages. As we first learned in
chapter 3
, green algae closely related to the extant genus
Cladophora
occur in 700–800-million-year-old shales in Spitsbergen. Indeed, microfossils interpreted as spores of planktonic green algae suggest that the “greening” of the oceans began at least a billion years ago.
Red algae also have a long Proterozoic history. The oldest fossils that can be compared with confidence to any living eukaryotes are beautifully preserved filaments found by Nick Butterfield in cherts about 1.2 billion years old from Somerset Island in arctic Canada (
plate 6a
and
b
). Each filament in Nick’s population consists of aspirin-shaped cells about 50 microns wide, aligned in a row. The cells are defined by thin, dark walls and united by a thicker but lighter outer wall layer. Cells are clearly grouped into pairs and pairs-of-pairs, providing evidence that these organisms grew by cell division within (rather than at the ends of) the filaments. Cells at the basal end are differentiated into holdfasts that anchored filaments to firm sediments on an ancient tidal flat. Another type of cell division is apparent in some filaments, and it is an unusual one; the aspirin-shaped cells sometimes divided repeatedly to form small reproductive bodies that resemble wedges of pie (
plate 6b
).
Collectively, these features ally the Somerset fossils with simple red algae (
plate 6c
and
d
). This means that the reds must have diverged from other eukaryotes, acquired photosynthesis (via endosymbiosis, as explained in the preceding chapter), and evolved a simple form of multicellularity by at least 1.2 billion years ago. Thus, both reds and greens appeared more than a billion years ago and diversified dramatically by 600 to 590 million years ago. Even heterokont algae born of secondary endosymbiosis may have differentiated early on. Fossils from the Lakhanda Formation in southeastern Siberia contain simple branching filaments comparable in morphological detail to the living heterokont
Vaucheria
. Lakhanda beds are cut by (and, so, are older than) igneous rocks dated at 1,003 ± 7 million years.
Other fossils strengthen the view that eukaryotes rose to prominence during the second half of the Proterozoic Eon. For example, microscopic fossils with distinctly eukaryotic spines or other ornamentation first appear in rocks about 1.2–1.3 billion years old and become increasing commonly as we ascend through the late Proterozoic record (
plate 6e
–
g
). More specifically, late Proterozoic biomarker molecules and (more controversially) microfossils record the presence of dinoflagellates, members of another major group of eukaryotes. Other biomarkers extracted from 750-million-year-old shales deep within the Grand Canyon suggest the presence of ciliate protozoans—phylogenetic cousins, actually, of the dinoflagellates.
Grand Canyon rocks document the early budding of one more branch on the eukaryotic tree. Distinctive vase-shaped microfossils entered my narrative early, in the discussion of Spitsbergen cherts and shales. Such fossils are common in upper Proterozoic rocks, often occurring in remarkable numbers, and nowhere are they more abundant than in the Grand Canyon. Working with exquisite populations preserved in carbonate nodules just beneath a bed of volcanic ash dated at 742 ± 6 million years, Harvard student Susannah Porter has demonstrated that these tiny vases were constructed by testate amoebas—amoeboid protozoans that live inside a minute shell, or test, of their own making (
figure 9.4
).
This discovery fascinates me because it sheds light on aspects of late Proterozoic ecology. Most of the microfossils discussed in this and previous chapters document photosynthetic organisms, either cyanobacteria or algae. Even the unusual fossils in Gunflint cherts were autotrophic, although they used chemical rather than solar energy to fuel cell growth. In contrast, the vase-shaped organisms were protozoans—heterotrophic eukaryotes that made their living by preying on other microorganisms. The vase-shaped microfossils, thus, tell us of growing ecological complexity in late Proterozoic oceans. Algae and cyanobacteria formed the nutritional base of ecosystems, providing food for untold bacteria. The testate amoebas dined on these algae and bacteria. Moreover, a few vases display hemispherical perforations likely made by other protozoans intent on eating
them
. So, by 750 million years ago, eukaryotes had begun to construct the complex food webs that today form a crown—intricate and unnecessary—atop ecosystems fundamentally maintained by prokaryotic metabolism.
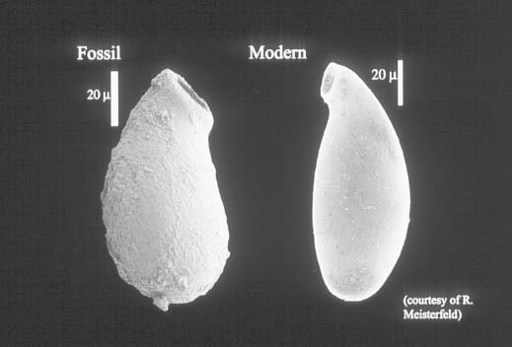
Figure 9.4.
Vase-shaped fossil from ca. 750-million-year-old rocks of the Grand Canyon compared with a modern testate amoeba. Note scale on photo. (Figure courtesy of Susannah Porter)
Spiny unicells, multicellular microfossils, compressed macrofossils, eukaryotic biomarker molecules—all can be used to trim the eukaryotic tree with ornaments of time (
figure 9.5
). They show that as the long Proterozoic Eon moved into its final phase, Earth was becoming a eukaryotic planet.
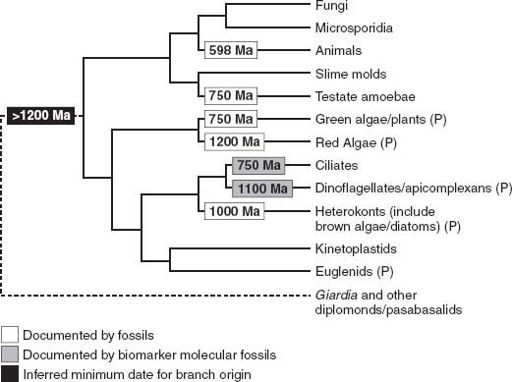
Figure 9.5.
The eukaryotic phylogeny first shown in
figure 8.2
, here trimmed with the dates of early eukaryotic fossils.
In contrast to the cyanobacteria discussed in previous chapters, most eukaryotic fossils don’t range through immense intervals of time. Rather, late Proterozoic algal and protozoan species appear in the record, persist for a discrete period, and then disappear, never to be seen again. The familiar pattern of punctuated equilibrium suggests evolutionary dynamics much like those of younger plants and animals, but enacted at a more leisurely pace—the stratigraphic ranges of many Proterozoic eukaryotes appear to be much greater than those of Phanerozoic species. In general, eukaryotic diversity increased through the late Proterozoic Eon and into the Cambrian, but the progress of diversification was halting, punctuated by several intervals of widespread extinction. As noted at the beginning of this chapter, at least some extinctions appear to be linked to latest Proterozoic climatic change.
There is a practical side to this evolutionary pattern—the eukaryotic microfossils in upper Proterozoic rocks can be used to tell time. Boris Timofeev, another of the Russian geologists charged with sorting out the Proterozoic rocks of Siberia, first recognized this potential. But it was
Gonzalo Vidal, a gregarious Spanish-cum-Swedish paleontologist from Uppsala University, who convinced initially skeptical geologists that Proterozoic eukaryotes came and went in a time-ordered fashion. Beginning with sandstones and shales exposed along the shores of Lake Vättern in south-central Sweden, Gonzalo discovered planktonic microfossils in upper Proterozoic rocks throughout Scandinavia. When he demonstrated how the fossils brought stratigraphic order to these rocks, the modern era of Proterozoic biostratigraphy was born.
As noted in the previous chapter, debate about eukaryotic phylogeny continues, but most disagreement focuses on the identity and character of the tree’s early branches. There is widespread agreement that much of the eukaryotic diversity seen today began to accumulate during a relatively short interval of rapid divergence. Paleontology seems to be telling us that this “big bang” of eukaryotic evolution began at least a billion years ago.
If that is true, why did this new type of biology take off so late in the evolutionary day? After all, as noted in
chapter 6
, sterane molecules extracted from 2.7-billion-year-old shales are thought to be molecular signatures of eukaryotic biology. If the course of eukaryotic evolution was set so early in life’s history, why should the domain (
our
domain!) have remained subservient to prokaryotes for a billion and a half years before spreading throughout the oceans? No one really knows, but we can think about four types of explanation. The 2.7-billion-year-old biomarker molecules record only one aspect of eukaryotic biology—the ability to make sterol compounds. Eukaryotes have many other distinguishing features, and perhaps the “complete” eukaryotic cell, with its distinctive genes, differentiated nucleus, cytoskeleton, and mitochondria, evolved much later. Alternatively, eukaryotes could have originated early but diverged much later, in the wake of some enabling environmental event. Or, late divergence might reflect biological innovation—sex is the one most commonly invoked. Of course, we must also ask whether the late Proterozoic radiation might be more apparent than real, reflecting a greater volume of rocks and better fossil preservation rather than increased biological diversity.
Shales that lie beneath the rubbly, tick-infested plains of Australia’s Top End discount the first and last explanations. Part of the middle
Proterozoic Roper Group, their age fixed by 1,492 ± 3–million-year-old volcanic rocks, these shales contain microfossils whose abundance and quality match the best preservation seen in upper Proterozoic rocks. Yet there are no spiny fossils like those in Guizhou, no tiny vases like the Grand Canyon and Spitsbergen populations, and no branching compressions comparable to those in younger shales from China or Spitsbergen. In short, Roper assemblages display little of the morphological variety that documents eukaryotic diversity in younger Proterozoic beds. There are, however, eukaryotic fossils.
Most Roper microfossils are large, compressed spheres, much like those in the broadly contemporaneous shales that flank Great Wall carbonates in northern Siberia; they are probably, but not demonstrably, eukaryotic. But one small population discovered by Emmanuelle Javaux, a Belgian postdoc in our lab, provides strong evidence of cytologically sophisticated eukaryotes in mid-Proterozoic oceans. The fossils are moderately large spheroids, about 30–150 microns in diameter, distinguished by one to as many as twenty long, slender tubes that arise from their walls (
plate 6e
). The tubes are irregular in number as well as position, and they sometimes branch. Similar shapes can be seen in some living protists, where tubes develop as extensions of spore walls, allowing reproductive cells that differentiate inside to escape and disperse. By analogy, then, the irregular tubes on the Roper fossils suggest microorganisms that could modify their shape during the lifetime of a single cell. Bacteria don’t do this very well, but eukaryotes are masters of the trade—their ability to form and re-form cell shape is conferred by the cytoskeleton, the dynamic internal scaffolding introduced in
chapter 8
. This being the case, Roper fossils tell us, not only that eukaryotic microorganisms were present nearly 1.5 billion years ago, but also that they already boasted some version of the sophisticated internal organization seen in living eukaryotes.