Life on a Young Planet (31 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

—T. S. Eliot
Little Gidding
F
OR THE LAST TIME
, we nose our rafts into the shore, eager to explore one final outcrop before the low drone of our approaching helicopter signals the end of another field season. Securing our boats to cobbles that line the stream, we take in the gray-gold cliffs that rise in front of us. The rocks look familiar, and they are. Having traveled across the globe and through 3 billion years of history, we have arrived once more at the Cambrian cliffs along the Kotuikan River. But now, as Eliot understood, we can see them with fresh eyes and so know them in new ways. Our peregrinations have, in fact, revealed the essential truth of Cambrian evolution: life has deep Precambrian roots, but the complex forms of Cambrian animals do not. There is nothing like the Cambrian until the Cambrian.
The Cambrian Explosion is the culmination of Precambrian evolution
but a departure from it, as well. Can we build an understanding that captures both the continuity and revolution of Cambrian biology?
To understand where life stood as the Proterozoic ended, and how it changed during the ensuing Cambrian, we need a map, provided—as ever in evolutionary biology—by phylogeny. I noted in
chapter 2
that anatomy and morphology early on revealed the relationships of some animal groups, but understanding how creatures as disparate as birds, clams, and tapeworms relate to one another challenged zoologists for many years. Embryology helped—for example, clams and polychaete worms share few features as adults, but many as larvae. But only in the age of molecular biology have the most difficult problems of animal phylogeny begun to yield.
Figure 11.1
shows the animal tree as currently understood. We can climb it, using structure, function, and development as handholds and introducing fossils as we ascend.
Animals are not just overgrown protozoans, and they probably wouldn’t have succeeded if they were. But what did metazoans do differently that enabled them to thrive on a crowded planet? Early branches of the tree highlight the achievements of multicellularity.
The closest known relatives of animals are the choanoflagellates, an
unusual group of colony-forming protozoans. Choanoflagellate cells sport a distinctive collar around their flagella, rather like Dutch burghers in a Rembrandt painting. The presence of similar collars on the food-gathering cells of sponges has long implicated choanoflagellates in animal origins. But not all sponge cells share this feature, underscoring a distinct difference between choanoflagellates and animals. In animals, the many cells that arise from a single fertilized egg vary in form and function, allowing metazoans to “multitask” in a way that protozoans can’t match.
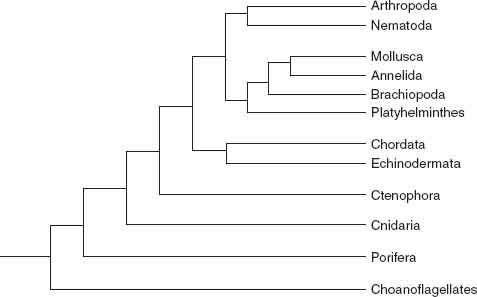
Figure 11.1.
Evolutionary relationships among animal phyla, as indicated by molecular phylogeny.
Sponges don’t simply produce different cell types; they array these differentiated cells into larger structures that facilitate food gathering and gas exchange. Sponges commonly grow into vases with hollow centers, porous walls, and an opening at the top. Collar cells that line the inner surface beat in unison, setting up water currents that bring in food particles and whisk away waste. A mosaic of flattened cells tiles the outer surface, while amoeba-like cells patrol the gelatinous zone in between, secreting fibrous proteins and, in some cases, mineralized skeletons of interlocking spicules.
Sponges undoubtedly diverged during the late Proterozoic, but they are not common in Ediacaran assemblages. Only with the evolution of mineralized skeletons near the Proterozoic-Cambrian boundary did sponges rise to paleontological prominence; skeletal fossils document dramatic Cambrian diversification within the phylum. Some early sponges secreted siliceous spicules—more than 90 percent of the five thousand sponge species found today fashion skeletons of silica, proteins (think of bath sponges), or both. Another group that persists to the present formed spicules, and sometimes massive skeletons, of calcium carbonate. Carbonate-secreting sponges called archaeocyathans were among the most diverse animals in Early Cambrian oceans, but mass extinction depleted their ranks halfway through the period, and for unknown reasons the group disappeared completely by the end of the interval. From ecosystem-dominant to evolutionary dead end in 20 million years—
sic transit gloria mundi.
Before climbing higher in the tree, we need to address a fundamental question: how do multicellular organisms differentiate their exquisitely complicated bodies as they grow? Complex multicellularity requires
cohesion among cells, communication between cells, and a genetic program to control cell differentiation during development. Cohesion keeps dividing cells from dispersing, making possible the precise spatial organization that underpins multicellular function. In seaweeds and plants, walls made of cellulose or other polysaccharides glue adjacent cells together. Animal cells, however, have no walls, so a battery of extracellular molecules must be deployed to make cells stick to one another; collagen, the protein that builds cartilage in humans, provides a prime example. Sponges produce a bevy of extracellular proteins that bind cells into vases; more complicated animals express similar proteins, but in greater variety.
In complex algae and land plants, thin strands of cytoplasm connect adjacent cells through tiny openings in their walls, providing a direct avenue for intercellular communication. In animals, molecular channels called gap junctions do much the same. Additionally, proteins embedded in the cell membrane bind chemical signals, setting off chain reactions that relay molecular messages to the nucleus. Cell surface proteins, thus, facilitate communication as well as cohesion, allowing groups of cells to function in a coordinated manner.
Communication is also key to animal development, the remarkable process by which fertilized eggs give rise to anatomically complex adults. When stressed, many single-celled eukaryotes cover themselves in a protective wall and suspend all but the most crucial cellular activities. That is, they differentiate into a distinct type of cell in response to signals from the environment. External signals also guide cell differentiation in animals, but in this case, the signals come from neighboring cells. A relatively small complement of genes, sometimes known as the developmental tool kit, coordinates the precise patterns of cell division, cell differentiation, and even programmed cell death that literally form hearts and minds. For the most part, these genes are not molecular carpenters assigned to build specific structures. They’re middle managers that receive instructions from one gene and pass them to the next. Programmed development, therefore, proceeds according to complex networks of gene interactions that collectively regulate growth. The genetic tool kit of sponges is relatively simple, those of complex animals like flies or mammals more richly elaborated. Similar regulatory networks
guide development in plants and algae, although many of the participating genes differ.
Sponges form one great limb of the animal tree; all other animals fall on the other.
1
More complicated animals, in turn, can also be divided into two major branches, the Cnidaria and the Bilateria (
figure 11.1
). We met these groups in
chapter 10
. Cnidarians comprise the jellyfish, corals, sea pens, and other taxa that provide structural analogues for many Ediacaran fossils; bilaterian animals, known mainly from trackways in Ediacaran sediments, today include an astonishing range of species from flatworms to whales.
As a group, cnidarians are distinctly more complicated than sponges—they have more types of cells, including muscle cells and a simple nerve network. Moreover, in cnidarians (and bilaterian animals), extracellular proteins bind cells into coherent sheets called epithelia that divide the animal body into compartments. Unlike sponges, therefore, cnidarians can form discrete tissues.
All cnidarians conform to a simple body plan—a hollow bowl or cylinder, with armlike tentacles around the opening (mouth). Two tissue layers that differentiate early in development line the inner and outer surfaces of the body, sandwiching gelatinous material in between (the “jelly” of jellyfish). The outer tissue, called
ectoderm
, contains muscle cells, nerves, and
cnidocytes
, specialized cells armed with tiny poisontipped harpoons, coiled and ready for action. (If you have ever been stung by a jellyfish, you have firsthand experience of cnidocytes.) The inner
endoderm
bristles with cells that secrete digestive enzymes. Cnidarians do not build complex organs that integrate several tissues, like the heart or stomach of a mammal. However, as explained in
chapter 10
, they gained complexity in another way—by differentiating functionally specialized individuals within colonies.
Collectively, muscle cells, nerves, and cnidocytes blazed a new trail in animal function. Sponges filter seawater to gather food particles, but cnidarians are predators, capturing prey with their harpoon-studded tentacles and stuffing it into their internal cavity for digestion. (As discussed earlier, reef corals and some other cnidarians have taken up farming, incorporating symbiotic algae into their tissues. Nonetheless, the Cnidaria fundamentally gather food by catching it.) Jellyfish and their relatives also
move
, further aiding the hunt.
Some protozoans catch and eat other cells, but with animals, predation took on an entirely new dimension. Protozoans might gobble cells singly or by the handful, but by filtering seawater with comblike organs, animals could catch them by the thousands and tens of thousands. And large size no longer provided safe haven. Animals, as well as microorganisms, had to avoid capture, and seaweeds had to cope with grazing. In effect, predatory animals became enormously important parts of the effective environment. The ensuing arms race between predator and prey has fueled evolution for more than 500 million years.
Many Ediacaran fossils probably relate to cnidarians, although most appear to represent early and extinct twigs on this branch. About ten thousand cnidarian species populate the modern oceans.
Remaining animal species—all 10 million of them,
2
including humans—belong to the Bilateria. Bilaterian animals differ from the Cnidaria in three fundamental ways. As explained in
chapter 10
, a single plane of symmetry divides the bilaterian body into left and right sides from head (more or less differentiated in most bilaterians) to tail. Moreover, three rather than two cell layers differentiate early in development—an ectoderm that contributes skin and nerve cells, an endoderm that gives rise to the digestive system, and an intervening layer called the mesoderm that differentiates into muscles and the reproductive system, among other things. Like cnidarians, bilaterian animals form tissues. Unlike cnidarians, however, bilaterians combine tissues
into complex organs, once again opening up new and diverse functional possibilities.
Cnidarians may have invented animal predation, but bilaterians perfected it. With organ systems came rapid swimming; muscular appendages to grasp and hold prey; mouths lined by mandibles, teeth, or rasping organs; sophisticated sensory organs including well-focused eyes; and, especially, brains able to coordinate the complex interactions of all these systems.
Increased predation intensified the need for protection. Some animals avoid predators by hiding. Others secrete poison. A third solution, discovered independently by many different groups, is armor—mineral-impregnated skeletons that protect against teeth and claws.
Cloudina
and
Namacalathus
show that at least a few late Proterozoic animals had lightly calcified coverings, but skeletons really took off in the Cambrian. The consequences for biology were significant, ratcheting up the evolutionary arms race and challenging predators to evolve structures that could pierce the defenses of prey. Mineralized skeletons also opened new functional pathways—for example, burrowing clams use their shells to dig into the sediment. Of course, the consequences for paleontology were also enormous. Skeletons preserve well in sedimentary rocks, increasing the likelihood that their makers will leave a fossil record. In fact, some geologists have argued that the Cambrian Explosion reflects the evolution of apparency—an explosion of fossils and not species. Such ideas, however, don’t withstand close scrutiny. Calcified fossils are common in latest Proterozoic reefs, but they don’t show any sign of the diverse and morphologically complex forms found in Cambrian and younger rocks (
plate 8
). Nor do late Proterozoic fossils replicated by calcium phosphate or compressed in shale even hint at the diversification to come. And, independently of any skeletons, trace fossils document a dramatic Cambrian diversification of animal behavior (
figure 11.2
). As emphasized by Stefan Bengtson, of the Swedish Museum of Natural History, skeletal evolution must be understood as part and parcel of the broader Cambrian diversification of animal life. Animals fashioned skeletons of calcium carbonate, calcium phosphate, silica, or just aggregated sediment particles—structural and biochemical innovations driven by the rise of sophisticated predators. (Of course, given the alternative strategies of speed, camouflage, and toxins, not all animals invested in mineralized skeletons. Only about one-third of the modern marine fauna make preservable skeletons, and in Cambrian oceans the percentage may have been even lower.)