Life's Ratchet: How Molecular Machines Extract Order from Chaos (31 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

While the force is large, the movement is tiny. It’s difficult to do a whole lot of useful work when moving in 0.5-nm steps. For this reason, the ATPase pocket in molecular motors is coupled to a mechanical amplifier—a part of the molecule that, like a lever, amplifies the motion of the ATPase pocket into a larger displacement. We encountered such mechanical amplification before when we discussed allostery. Energy conservation demands that the force is reduced proportionally to the increase in displacement. If I want to generate ten times the motion, specifically, 5 nm, the force I could generate would be ten times smaller, or only 11.6 pN. In reality, the efficiency of these motors is only about 60 percent (which is still very good), and amplified motions range from 5 to 36 nm, while forces range from 1 to 10 pN. This really does seem small, but even 1 pN is enough to move molecular cargo around, especially if a number of motors work together.
TWEEZERS OF LIGHT
Measuring the displacements and forces generated by molecular motors sounds like an incredibly tricky proposition. After all, these motors are only a few tens of nanometers large, and we have seen how incredibly tiny a nanometer is. You could hardly put one of these tiny molecules on a leash and measure how much it tugs. Or could you?
In 1993, Steven Block and his research group at Harvard attached kinesin molecular motors to small silica beads, using small molecular leashes. The beads were then captured in a trap made of light—a laser trap. Light is made of particles, or photons, and these photons have momentum and can impart an impulse on a particle. Many photons together can create pressure. In a laser trap, high-intensity laser light is focused such that the pressure of the photons creates a minute inward force, a force that will keep a small particle from escaping the center of the laser focus. The force is only about 1 to 10 pN, but the weight of a 600-nm diameter bead, as used in Block’s study, is only 0.002 pN, so the light pressure is more than sufficient to suspend and hold the bead (
Figure 7.3
). Laser traps are amazing devices: It is relatively easy to move light with lenses and mirrors and thus the focus and the trapped bead can be moved together in three dimensions. The laser trap thus acts like highly sensitive tweezers—tweezers that can pick up nanometer-size beads and single molecules attached to the bead.
In Block’s experiment, he and his students picked up a bead out of a suspension of numerous beads. Each bead was decorated with kinesin molecules. Once a bead was captured, it was lowered onto a microtubule, the protein track on which kinesin molecular motors move, which was fixed to a glass slide. Block’s group combined their laser trap with interferometry, using two separate light beams to illuminate the object. From the interference of the light scattered by the bead, they could determine the motion of the bead with high accuracy.
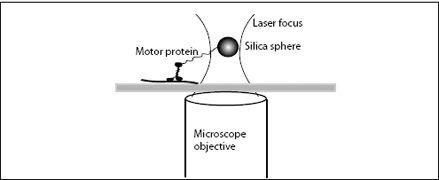
FIGURE 7.3.
A laser tweezer measurement of the motion and forces of a transport motor protein.
Motion came from two sources: thermal noise (the molecular storm), and the deliberate motion of the motor proteins. As the kinesin molecule moved along its track, it pulled the bead along with it. As the bead moved to the edge of the light trap, the force increased from 0 to 1.5 pN. Tracking the motion and simultaneously measuring the force, Block and his colleagues were able to measure the speed and step size of a single kinesin motor as a function of the load it had to pull. Once the force became too much, the motor detached from the track, the bead was pulled back into the center of laser focus, the kinesin motor attached again, and the measurement was repeated.
At low ATP concentrations, Block found that the bead was pulled by the motor with a speed of 50 nm per second. Applying statistics to the noisy data, they found that kinesin took 6- to 8-nm-size steps, and therefore the kinesin seemed to step about six to eight times every second. They also found that the kinesin was an almost perfect rectifier of thermal motion, almost never stepping backward.
As they raised the ATP concentration, the motor became faster, finally reaching a maximum speed of 500 nm per second. At this speed, the little molecule would traverse an entire cell in about a minute. Now, Block and his students gave the little motor a challenge: Increasing the laser intensity, they created a higher trapping force and made the little motor pull against it. They found that the kinesin motor could pull against forces as high as 5 pN. By comparison, the force needed to pull a 600-nm bead at 500 nm per second through water is about 0.03 pN. Thus, this little 50-nm-long motor can easily pull an object many times its size at a pretty fast clip through the cell. It is a molecular worker ant.
Since Block’s pioneering work, the use of laser tweezers to study molecular machines has advanced considerably. Noise is much lower, feedback loops are used to control force or position, and multiple traps can manipulate single molecules in myriad ways. Sixteen years later, Block and his coworkers have been able to follow the movement of a single leg of kinesin by attaching the bead to one of the heads of the motor protein via a DNA tether. Using the DNA tether, they gently pulled on the leg to see if it was bound to the microtubule or if it was dangling freely. In addition to the amazing skill involved in chemically attaching a DNA molecule to a specific 10-nm part of a 50-nm molecule and then attaching the DNA to
a bead trapped in a beam of laser light, the data obtained greatly surpassed Block’s 1993 data. Back then, Block and his students had to use statistical analysis to extract meaningful information from the noisy traces of the bead’s movement. In 2009, every step of the molecules head was clearly seen with nanometer resolution.
FLUORESCENCE MAGIC
The amazing feats of molecular motors were being discovered long before laser tweezers were developed. The first true single-molecule techniques were developed in the 1980s. These methods were based on attaching fluorescent molecules to the motor protein. Fluorescence is the emission of characteristic colors of light in response to a material’s being excited by light of higher energy. When high-energy photons hit a fluorescent molecule, they cause the electrons in the molecule to jump to a higher orbit (or energy state). The molecule is now in an excited state, but after some time, the electrons will relax back to a lower energy state. The difference in energy between the excited and the lower energy state is emitted as light of a characteristic energy and, therefore, a characteristic color. A well-known example of fluorescence is the eerie glow of your T-shirt when you enter a dance club with black lights. The ultraviolet light produced by black lights is highly energetic and can excite the molecules in your clothes. As the electrons fall back down, they produce a bluish visible light—this is fluorescence.
Using sensitive sensors, researchers could detect the light from a single fluorescence molecule. In this way, they could follow the motions of single molecular motors in an optical microscope, even if the motors themselves were too small to see. All the scientists had to do was follow the light emitted by the fluorescent marker attached to the motor.
This ability to detect amounts of light down to single photons has produced a cottage industry of fluorescence-based single-molecule techniques. As is customary in science, they all have fancy acronyms, from TIRF to FRET, FCS, and SHRIMP. A pioneer in using fluorescence techniques to study molecular motors is Jim Spudich at Stanford University. In 1983, he developed what is now known as the bead assay. Spudich and his students attached myosin molecules to fluorescently labeled beads
(much as Block attached kinesin to beads ten years later for his laser trap). He then floated the beads onto a bed of actin filaments. As soon as the myosin molecules contacted the actin and were properly fed with ATP, the fluorescent beads started to move around like microscopic fireflies. In 1986, Spudich did the opposite. This time he attached myosin to a substrate and then floated fluorescently labeled actin filaments on top of the motors. Again, upon proper feeding, the myosin molecule started to push the actin filaments around, creating a wiggling spectacle of gliding filaments. This gliding-filament assay is what I saw twenty-five years later at the biophysical meeting, and it was still as fascinating as when it was first created. Watching the filaments move around like glowing worms reminded me that the motion inherent in life, so mysterious to the Greeks and early-twentieth-century researchers alike, was now without a doubt explained by the amazing motions of mere molecules.
HOW MOTORS STEP: KINESIN AND MYOSIN V
Kinesin-1 and myosin V (for some reason, the kinesin researchers like to use Arabic numerals, and the myosin researchers Roman) are the quintessential walking motors. Both these proteins are processive, so they can walk long distances without falling off their tracks. Kinesin walks on microtubules, while myosin strolls along actin filaments. Scores of detailed studies involving fluorescence, AFM, laser tweezers, X-rays, electron microscopy, and the use of mutated motor proteins have brought us very close to deciphering a detailed picture of how these amazing walking molecules move. Structural studies have shown that the ATP binding site, or
motor domain
—the part of the motor that binds its track and binds ATP—is very similar in both proteins. This indicates that the two come from a common ancestor. However, somewhat surprisingly, the way the two molecules move is completely different. While myosin V strides, kinesin-1 waddles. Moreover, the two motor proteins use their ATP for completely different purposes, as we will see.
The details of how the motors walk are still controversial. Let’s look more closely at kinesin, or, rather, a specific kind of kinesin, called kinesin-1. We briefly observed kinesin in
Chapter 6
, to gain some understanding of the complex issues. Kinesin-1 consists of two motor domains (or
heads
),
which attach to, and detach from, the microtubule. The heads are linked through a short neck linker, which does not allow a huge amount of flexibility. Thus kinesin is condemned to waddling—it’s like how you might walk if your pants had slid down to your ankles. Beyond the neck linker is the
coiled coil
(that’s not a typo—it’s a coil of two coils), which is the longest part of the molecule. The coiled coil is crowned by a cargo-binding domain, whose function is self-explanatory (
Figure 7.4
).
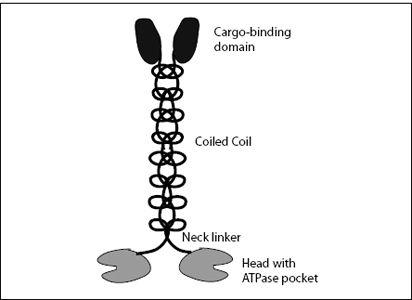
FIGURE 7.4.
Structure of a kinesin-1 molecule. At the bottom are the two heads on which the molecule waddles along a microtubule filament. Each head has a pocket to bind and hydrolyze ATP. The two heads are held together by a short neck linker. On top of this is a coiled-coil stalk, which ends with the cargo-binding domain.
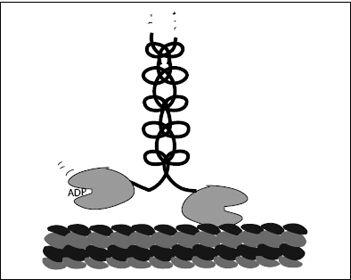
To follow the motion of a kinesin molecule, we have to start at some point along its cycle. Let’s start just as the head in front is empty, binding neither ATP nor ADP but bound to the microtubule. At the same moment, the trailing head has an ADP bound in its ATPase pocket. Block’s 2009 experiment suggests (for now) that the ADP-bound head’s affinity to the microtubule is very low. In other words, the trailing head is freely dangling, while the leading, empty head is firmly attached to the microtubule. Block calls this configuration the waiting state (
Figure 7.5
).