The Case for Mars (38 page)

On Mars there is an atmosphere thick enough to protect crops grown on the surface from solar flares. Therefore, thin-walled inflatable plastic greenhouses protected by unpressurized UV-resistant hard-plastic shield domes can be used to rapidly create crop land on the surface. Even without the problems of solar flares and a month-long diurnal cycle, such simple greenhouses would be impractical on the Moon because they would create unbearably high temperatures. On Mars, in contrast, the strong greenhouse effect created by such domes would be precisely what is necessary to produce a temperate climate inside. Such domes up to 50 meters in diameter are light enough to be transported from Earth initially, and later on they can be manufactured on Mars out of indigenous materials. Because all the resources to make plastics exist on Mars, networks of such 50- to 100-meter domes could rapidly be manufactured and deployed, opening up large areas of the surface to both shirtsleeve human habitation and agriculture. That’s just the beginning, because, as we shall see in Chapter 9, it will eventually be possible for humans to substantially thicken Mars’ atmosphere by forcing the regolith to outgas its contents through a deliberate program of artificially induced global warming. Once that has been accomplished, the habitation domes could be virtually any size, as they would not have to sustain a pressure differential between their interior and exterior. In fact, once that has been done, it will be possible to raise specially bred crops outside the domes.
The point to be made is that unlike colonists on any other known extraterrestrial body, Martian colonists will be able to live on the surface, not in tunnels, and move about freely and grow crops in the light of day. Mars is a place where humans can live and multiply to large numbers, supporting themselves with products of every description made out of indigenous materials. Mars is thus a place where an actual civilization, not just a mining or scientific outpost, can be developed. And significantly for interplanetary commerce,
Mars and Earth are the only two locations in the solar system where humans will be able to grow crops for export
.
INTERPLANETARY COMMERCE
Mars is the best target for colonization in the solar system because it has
by far the greatest potential for self-sufficiency. Nevertheless, even with optimistic development of robotic manufacturing techniues, Mars will not have the labor power required to make it fully self-sufficient until its population numbers in the millions. Thus, for centuries it will be necessary, and forever it will be desirable, for Mars to be able to import specialized manufactured goods from Earth. These goods can be fairly limited in mass, as only small portions (by weight) of even very high-tech goods are actually complex. Nevertheless, these smaller sophisticated items will have to be paid for, and the high costs of Earth-launch and interplanetary transport will greatly increase their price. What can Mars possibly export to Earth in return?
It is this question that has caused many to deem Mars colonization intractable, or at least inferior in prospect to the Moon. For example, much has been made of the fact that the Moon has indigenous supplies of helium-3, an isotope not found on Earth and which could be of considerable value as a fuel for second-generation thermonuclear fusion reactors. Mars has no known helium-3 resources. On the other hand, because of its complex geologic history, Mars may contain concentrated mineral ores, with much greater concentrations of precious metal ores readily available than is currently the case on Earth—because the terrestrial ores have been heavily scavenged by humans for the past five thousand years. In a paper I co-authored with David Baker a few years ago, I showed that if concentrated supplies of metals of equal or greater value than silver (such as silver, germanium, hafnium, lanthanum, cerium, rhenium, samarium, gallium, gadolinium, gold, palladium, iridium, rubidium, platinum, rhodium, europium, and a host of others) were available on Mars, they could potentially be transported back to Earth for a substantial profit.
37
Reusable Mars-surface-based single-stage-to-orbit vehicles such as NIMFs (discussed in Chapter 7) could haul cargoes
to Mars orbit for transportation to Earth via either cheap expendable chemical stages manufactured on Mars or reusable cycling solar or magnetic sail-powered interplanetary spacecraft. (These advanced propulsion systems are discussed in the focus section at the end of this chapter.) The existence of such precious metal ores, however, is still hypothetical.
But there is one commercial resource that is known to exist ubiquitously on Mars in large amounts—deuterium. Deuterium, the heavy isotope of hydrogen, occurs as 166 out of every million hydrogen atoms on Earth, but comprises 833 out of every million hydrogen atoms on Mars. Deuterium is the key fuel not only for both first- and second-generation fusion reactors, but it is also an essential material for the nuclear power industry today. If you have enough deuterium, you can moderate a nuclear fission reactor with “heavy water” instead of ordinary “light water,” and such a heavy-water moderated reactor can run on natural uranium, with no enrichment required. Canadian-made nuclear power reactors known as “CANDUs” work on this principle today. The problem however, is that you have to electrolyze 30 tonnes of ordinary “light” water to produce enough hydrogen to make one kilogram of deuterium, and unless you have a lot of very cheap hydroelectric power to burn, the process is prohibitively expensive. (This is why in World War II the German atomic bomb project had to conduct its heavy water production near the large Norwegian hydroelectric dams at Vemork. When Norwegian resistance commandos and U.S. B-17s wrecked the place in a series of raids in 1943, Germany’s nuclear program was effectively destroyed.) Even with cheap power, deuterium is very expensive; its current market value on Earth is about $10,000 per kilogram, roughly 50 times as valuable as silver or 70 percent as valuable as gold. This is in today’s pre-fusion economy. Once fusion reactors go into widespread use deuterium pricesill increase. As discussed in the previous chapters, the Mars base will be using most of its power in water electrolysis to drive its various life-support and chemical synthesis processes. If a deuterium/hydrogen separation stage is applied to the hydrogen produced by the electrolysis operations prior to recirculating it back into the chemical reactors, then every 6 tonnes of Martian water electrolyzed can provide about one kilogram of deuterium as a by-product. Each person on Mars will require about 10 tonnes of water electrolysis per (Earth) year. If the amount of water electrolysis supporting the various materials-processing operations is twice this, a total of 6 million tonnes per year of water electrolysis will be required by a 200,000 person Mars colony. This will result in the production of 1
,000 tonnes of deuterium per year, enough to produce 11 terrawatts (TW) of electricity, or about what the entire human race consumes today. At current deuterium prices this represents an annual export income potential of $10 billion—a figure comparable to a nation of much greater size on Earth. (For example, New Zealand booked $11.2 billion of gross exports in 1994, yet is a nation of 3.4 million.) At the current average rate of 5 cents/kilowatt-hour for electricity, the total value of the power produced on Earth as a result would total about $5 trillion per year.
Ideas may be another possible export for Martian colonists. Just as the labor shortage prevalent in colonial and nineteenth-century America drove the creation of “Yankee ingenuity,” so the conditions of extreme labor shortage combined with a technological culture will tend to drive Martian ingenuity to produce wave after wave of invention in energy production, automation and robotics, biotechnology, and other areas. These inventions, licensed on Earth, could finance Mars even as they revolutionize and advance terrestrial living standards as forcefully as nineteenth-century American invention changed Europe and ultimately the rest of the world as well.
Inventions produced as a matter of necessity by a frontier culture can make Mars rich, but invention and direct export to Earth are not the only ways that Martians will be able to make a fortune. The other route is via trade in support of mining operations in the asteroid belt, the band of small, mineral-rich bodies lying between the orbits of Mars and Jupiter.
To understand this, it is necessary to consider the energy relationships between the Earth, Moon, Mars, and the main asteroid belt. The asteroid belt enters into the picture here because it is known to contain vast supplies of very high grade metal ore in a low-gravity environment that makes it potentially easy to export to Earth.
29
For example, John Lewis of the University of Arizona has considered the case of a run-of-the-mill asteroid just one kilometer in diameter. This asteroid would have a mass of two billion tonnes, of which 200 million tonnes would be iron, 30 million tonnes would be high-quality nickel, 1.5 million tonnes would be the strategic metal cobalt, and 7,500 tonnes would be a mixture of platinum group metals whose average value at current prices would be in the neighborhood of $20,000 per kilogram. That adds up to $150 billion for the platinum alone. There is little doubt about this, for we have lots of samples of asteroids in the form of meteorites. As a rule, meteoritic iron contains between 6 and 30 percent nickel, between 0.5 and 1 percent cobalt, and platinum group metal concentrations at least 10 times the best terrestrial ore. Furthermore, since the asteroids also contain a good deal of carbon and oxygen, all of these materials can be separated from the asteroid and from each other using variations of the carbon monoxide-based chemistry we discussed in Chapter 7 for refining metals on Mars Maright="0em">
There are about 5,000 asteroids known today, of which about 98 percent are in the “Main Belt” between Mars and Jupiter, with an average distance from the Sun of about 2.7 astronomical units, or AU. (The Earth is 1.0 AU from the Sun.) This Main Belt group includes all the known asteroids residing within the orbit of Jupiter with diameters greater than 10 kilometers, hundreds larger than 100 kilometers, and one as large as 914 kilometers. Except for a few tiny objects that travel closer to the Sun than the Earth and a handful that have been spotted beyond Jupiter, the remaining 2 percent, all small, have orbits lying between Earth and Mars. The 2 percent figure, however, greatly overstates the proportion of such “near Earth” asteroids to Main Belters, because their relative closeness to the Earth and Sun makes them much easier to see. A reasonable estimate would be that the Main Belt asteroids outnumber the near-Earth group at least a thousand to one. Of the “near Earth” asteroids, about 90 percent orbit closer to Mars than to the Earth.
As should be clear from Lewis’s example, these asteroids collectively represent enormous economic potential. While much has been made recently of the importance of the near-Earth group (especially due to gradual realization that if we don’t develop some serious space-faring capabilities, one of them is likely to wipe out the human race when it impacts our planet someday), the relative numbers of the two classes make it clear that for mining purposes, the real action is going to be in the Main Belt.
Miners operatin
g among the asteroids will be unable to produce most of their necessary supplies locally. There will thus be a need to import food and other necessary goods either from Earth or Mars. As shown in the table below, Mars has an overwhelming positional advantage as a location from which to conduct such trade. This advantage results from the fact that the rocket propulsion ΔVs required to reach the asteroid belt from Mars are much less than those from Earth, and as a result, the mass ratio (a spacecraft’s fully fueled mass divided by its dry mass) required of spacecraft leaving Mars is also much less.
TABLE 8.1
Transportation in the Inner Solar System
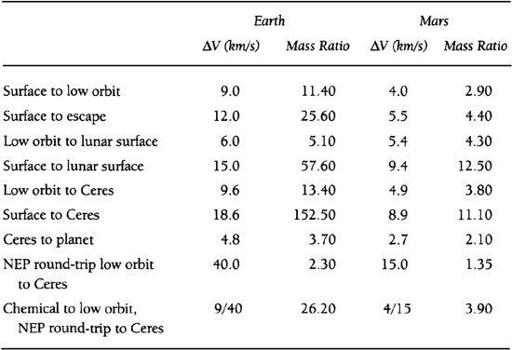
In
Table 8.1
, Ceres is chosen as a typical Main Belt asteroid destination, as it is the largest asteroid and positioned right in the heart of the Belt. You’ll notice, however, that I also give the Earth’s Moon as a potential port of call. Despite the fact that it is much closer to Earth physically, we can see that from a propulsion point of view, it is much easier to reach the Moon from Mars than it is from the Earth! That is, the required mass ratio is only 12.5 going from Mars to the Moon, while it is 57.6 from Earth. This would be even more forcefully the case for travel from either Earth or Mars to nearly any “near-Earth” asteroid as well.
All the entri
es presented in
Table 8.1
except the last two are based upon a transportation system using methane/oxygen (CH
4
/O
2
) engines with a specific impulse (Isp) of 380 seconds and ΔVs appropriate for trajectories employing high-thrust chemical propulsion systems. These were chosen because methane/oxygen is the highest performing space-storable chemical propellant, and can be manufactured easily on Earth, on Mars, or on a carbonaceous asteroid. Hydrogen/oxygen propellants, while offering a higher Isp (450 seconds), are not storable for long periods in space. Moreover, a f an unsuitable propellant for a cheap reusable space transportation system, since its costs exceed methane/oxygen propellant by more than an order of magnitude and its bulk makes it very difficult to transport to orbit in any quantity using reusable single-stage-to-orbit (SSTO) vehicles (thus ruling it out for true cheap surface-to-orbit systems). The last two entries in the table are based upon nuclear electric propulsion (NEP) using argon propellant, available at either the Earth or Mars, with an Isp of 5,000 seconds for in-space propulsion, with methane/oxygen used to reach low orbit from the planet’s surface. Such SSTO and NEP systems, while somewhat futuristic today represent a conservative baseline for interplanetary transportation technology in the period we are discussing.