The Case for Mars (42 page)

Humans are only the most recent practitioners of this art. Starting with our earliest civilizations, we used irrigation, crop seedings, weeding, domestication of animals, and protection of their herds to enhance the activity of those parts of the Earth most efficient in supporting human life. In so doing, we have expanded the biospheric basis for human population, which has expanded our numbers and thereby our power to change our environment to support a continued cycle of exponential growth. As a result, we have literally remade the Earth into a place that can support billions of people, a substantial fraction of whom have been sufficiently liberated from the need to toil for daily survival—such that some can now look out into the night sky for new worlds to conquer.
Some people consider the idea of terraforming Mars heretical—humanity playing God. Yet others would see in such an accomplishment the most profound vindication of the divine nature of the human spirit, exercised in its highest form to bring a dead world to life. My own sympathies are with the latter group. Indeed, I would go farther.
I would say that failure to terraform Mars constitutes failure to live up to
our human nature and a betrayal of our responsibility as members of the community of life itself
. Today, the living biosphere has the potential to expand its reach to encompass a
whole new world. Humans, with their intelligence and technology, are the unique means that the biosphere has evolved to allow it to make that land grab, the first among many. Countless beings have lived and died to transform the Earth into a place that could create and allow human existence. Now it’s our turn to do our part.
So let’s pose the question once again: Can we
transform
Mars to make it fully habitable?
Let’s consider the matter. Despite the fact that Mars today is a cold, dry, and probably lifeless planet, it has all the elements required to support life: water, carbon and oxygen (as carbon dioxide), and nitrogen. The physical aspects of Mars, its gravity, rotation rate, axial tilt, and distance from the Sun, are close enough to those of Earth to be acceptable. Mars does want in one area, though: It does not have much of an atmosphere.
Earth’s atmospheric pressure at sa level is 14.7 pounds per square inch, or approximately 1 bar. (A “bar” is simply a unit for measuring pressure. “Bar” and “millibar,” one-thousandth of a bar, are commonly used in meteorology and will be used in our discussion of terraforming.) Mars’ current carbon dioxide atmosphere is less than 1 percent that of Earth’s at sea level, varying between 6 and 10 millibars (mbar or mb). However, we know that Mars’ atmosphere was once much thicker than it is now. The channels that snake along the Martian surface provide evidence that liquid water once coursed over the planet, and liquid water can exist only within a certain temperature and pressure range. At sea level on Earth, the temperature range is between 0° centigrade—the freezing point of water—and 100° centigrade—the boiling point of water. For water to run across the surface of Mars, the atmospheric pressure and temperature had to be greater than they are today.
Though Mars’ atmosphere currently is rather thin, most researchers believe that there are enough reserves of carbon dioxide on the planet to substantially thicken it. Some of this carbon dioxide exists in frozen form as “dry ice” and makes up the south polar cap. Additional reserves are trapped within its regolith, the loose surface material that overlays the planet. (“Regolith” is an astrogeologist’s term for dirt, and applies to any planetary body. “Soil” refers to Earth’s regolith.) Relea
sing all of this carbon dioxide would greatly thicken the atmosphere, possibly to the point where its pressure would be about 30 percent that of Earth, or 300 mbar (almost one-third of a bar). Heating the planet will cause these vast reservoirs of trapped carbon dioxide to emerge. This is not just theory: We know for a fact that Mars’ temperature and atmospheric pressure vary as the planet cycles between its nearest and furthest positions from the Sun over the course of a Martian year. In fact, as Mars warms and cools over the course of a year, its atmospheric pressure varies by plus or minus 20 percent compared to the average value on a seasonal basis.
We cannot, of course, move Mars to a warmer orbit. But we do know another way to heat a planet, one that we apparently have unwittingly practiced on Earth for perhaps the past century. That is to release or produce gases that will trap infrared radiation—the Sun’s heat—and thereby warm the planet. On Earth it’s called the “greenhouse effect” and has resulted from the introduction of carbon dioxide released from fossil fuel burning, as well as other greenhouse gases produced by industry. Call it terraforming or call it greenhousing, but the same can happen on Mars. An atmospheric greenhouse could be created on Mars in at least three different ways: by warming selected areas of the planet to release large reservoirs of the native greenhouse gas, carbon dioxide; by establishing factories on Mars to produce very powerful artificial greenhouse gases such as halocarbons (“CFCs”); or by releasing bacteria that could produce natural greenhouse gases more powerful than carbon dioxide (but much less so than halocarbons) such as ammonia or methane, once acceptable living conditions for bacteria were produced on Mars by one of the other methods.
While the concept of terraforming Mars may seem fantastic, the concepts supporting the notion are straightforward. Chief among them is that of positive feedback, a phenomenon that occurs when the output of a system enhances what is input to the system. For a Mars greenhouse effect, we find a positive feedback system in the relationship between atmospheric pressure—its thickness—and atmospheric temperature. Heating Mars will release carbon dioxide from the poaps and from Martian regolith. The liberated carbon dioxide thickens the atmosphere and boosts its ability to trap heat. Tr
apping heat increases the surface temperature and, therefore, the amount of carbon dioxide that can be liberated from the ice caps and Martian regolith. And that is the key to terraforming Mars—the warmer it gets, the thicker the atmosphere becomes; and the thicker the atmosphere becomes, the warmer it gets.
In the sections below, we’ll see how this system can be modeled, and present the results of calculations using such a model. These results give very strong support to the belief that humans will be able to effect radical improvements in the habitability of the Martian environment in the course of the twenty-first century. We can, indeed, transform Mars.
TERRAFORMING CALCULATIONS
As I’ve noted, Mars is awash with carbon dioxide, a premier greenhouse gas, but much of that is trapped at the poles in frozen form, or locked in the planet’s regolith. Both sources of carbon dioxide will help greenhouse Mars, but it’s the frozen carbon dioxide at the poles that will initiate the process.
In recent computational studies Chris McKay and I utilized Mars climate models to reveal that a small but sustained change in temperature at the Martian south pole—just 4°C—can initiate a runaway greenhouse effect in the polar region that will result in the evaporation of the polar cap. (For those wanting to delve into the fine points, I’ve included a technical note at the end of the chapter that details the model we used as a basis for this discussion of terraforming.) As the cap evaporates, global atmospheric temperature and pressure will rise, and that, in turn, will initiate the liberation of huge quantities of carbon dioxide locked in the regolith. In short, a modest 4°C rise in temperature at the south pole can globally raise temperatures by tens of degrees and transform a 6 millibar atmosphere into one measured in hundreds of millibars.
Raising the temperature of the south pole by just 4°C hardly seems enough of a change to trigger such a planetary transformation, but it’s akin to removing just one apple from the bottom of a carefully stacked pyramid at the grocery store. Someone worked
long and hard to arrange those apples in a state of delicate balance, of equilibrium. It doesn’t take much to knock that equilibrium askew. So it is with Mars’ south polar cap. Frozen carbon dioxide—dry ice—forms the cap. Carbon dioxide can be characterized by a measure known as “vapor pressure,” which is a measure of the tendency of a substance to change into a gaseous or vaporous state. Temperature alone affects the vapor pressure of a substance, and as you turn up the heat on a substance, you raise its vapor pressure—it will change into a vapor or gas more vigorously. The vapor pressure of carbon dioxide at 147° Kelvin is 6 millibars—the current conditions found at Mars’ south pole. (Kelvin degrees are chilly; if you have the temperature in degrees Kelvin, you need to subtract 273 to translate it into degrees centigrade. Therefore, 273°K equals 0°C, or 32°F. The temperature at Mars’ south polar cap, 147°K, is -126°C or -195°F.) This is a state of equilibrium for the polar cap. So long as the pole remains at that temperature, it’s difficult for the carbon dioxide pressure to get much above 6 millibar because excess carbon dioxide will simply condense out of the atmosphere and return to its frozen, dry ice form.
Now, what if we increased the temperature at the pole artificially? Later I will detail how this could be accomplished uurrent arge, orbital mirrors to concentrate sunlight on the pole, but, for the moment, let’s say that we have started to artificially heat up the pole. As a consequence of raising the temperature, the vapor pressure of carbon dioxide will increase, and therefore more carbon dioxide will evaporate from the cap into the atmosphere. Vapor pressure—the tendency for a substance to transform into a gas or vapor—and atmospheric pressure—the actual weight of an atmosphere over the surface—are two very different concepts, but we can say that as the vapor pressure of carbon dioxide rises at the pole, Mars’ global atmospheric pressure will rise as a consequence of the carbon dioxide being pumped into the atmosphere as the cap evaporates. The vapor pressure of carbon dioxide at any temperature is a known piece of scientific information—you can look it up in a chemical handbook—and what holds for carbon dioxide on Earth will hold for carbon dioxide on Mars. The greenhousing capability of a layer of carbon dioxide gas in a planetary atmosphere is also known, albeit with somewhat les
s precision, so it is possible to estimate with reasonable accuracy how much the temperature on Mars will increase as a result of the thickening of its atmosphere. With this basic understanding of the conditions at the pole, of the vapor pressure and its relation to temperature, we can now venture forward into the nitty-gritty calculations that reveal how we can jump start the terraforming of Mars.
FIGURE 9.1
Mars polar cap/atmosphere dynamics. Current equilibrium is at point A. Raising polar temperatures by 4°K would drive equilibria A and B together, causing runaway heating that would lead to the elimination of the cap
.
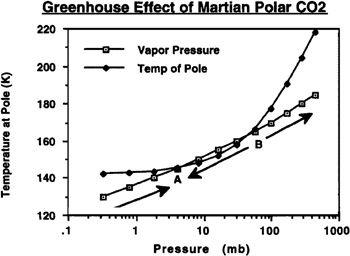
To start, take a look at
Figure 9.1
. In this figure, you can see the results of a model developed by McKay and me when applied to the situation at Mars’ south polar cap, where we believe there may exist enough frozen carbon dioxide to give Mars an atmosphere on the order of 50 to 100 mbar. I have plotted the polar temperature as a function of the atmospheric pressure, and the vapor pressure as a function of the polar temperature. Note the two points, A and B, where the curves cross. Each is an equilibrium point where Mars’ mean atmospheric pressure (P—the atmospheric pressure at Mars’ mean surface elevation, in millibars) and the polar temperature (T—in degrees Kelvin) given by these two curves
are mutually consistent. However, A is a stable equilibrium, while B is unstable. This can be seen by examining the dynamics of the system wherever the two curves do not coincide. Whenever the temperature curve lies above the vapor pressure curve, the system will move to the right, or towards increased temperature and pressure; this would represent a runaway greenhouse effect. Whenever the temperature curve lies below the pressure curve, the system will move to the left, or toward decreased temperatures and pressure; this would represent a runaway “icebox effect.” Mars today is at point A, with 6 millibar of pressure and a temperature of about 147° Kelvin at the pole.
Now consider what would happen if someone artificially increased the temperature of the Martian pole by several degrees Kelvin. As the temperature is increased, the whole temperature curve would move upwards, causing points A and B to move towards each other until they met. If the temperature increase were 4°K, the temperature curve would move far enough up the graph to lie above the vapor pressure curve everywhere. The result would be a runaway greenhouse effect that would cause the entire pole to evaporate, perhaps in less than a decade. Once the pressure and tempeature have moved past the current location of point B, Mars will be in a runaway greenhouse condition even without artificial heating, so if later the heating activity were discontinued, the atmosphere will remain in place.
As the polar cap evaporates, the dynamics of the greenhouse effect caused by the reserves of carbon dioxide held in the Martian regolith come into play. These reserves exist primarily in the high latitude regions, and by themselves could be enough to give Mars a 400 mbar atmosphere. We can’t get them all out, however, because as they are forced out of the ground by warming, the regolith becomes an increasingly effective “dry sponge” acting to hold them back. Unfortunately, we face a major unknown at this point—the amount of energy or temperature change required to release the carbon dioxide from Martian regolith. We’ll call this unknown the temperature of desorption (T
d
) and estimate it at 20° Kelvin, though later we will vary its value to see how our model plays out. The dynamics of the atmosphere and regolith are shown in
Figure 9.2
. This figure displays the regolith-created atmospheric pressure on Mars (denoted “regolith pressure” in the figure) as a function of the regolith temperature, T
reg
. (T
reg
is the average of the planet’s regolith temperature with different regions weighted in accordance with how much adsorbed gas they can hold at their own local temperature. Because colder soil holds more CO
2
, T
reg
tends to be representative of the temperatures in Mars’ near-Arctic or near-Antarctic regions.) The figure also displays a plot of the regolith temperature as a function of the carbon dioxide pressure in the atmosphere. To arrive at these plots, I have assumed that releasing all available current polar carbon dioxide reserves would boost atmospheric pressure 100 millibars, and releasing all of the regolith carbon dioxide reserves would boost atmospheric pressure 394 millibars. Thus, together with the 6 millibars already in the atmosphere, Mars in this example is assumed to have a total carbon dioxide inventory of 500 millibars.