The Case for Mars (44 page)

The rate at which gas comes out of the regolith will be in direct proportion to the rate at which a temperature increase that we create on the surface of Mars can penetrate into the ground. We can get a pretty good estimate for this by assuming that Martian regolith is a lot like dry soil on Earth, with may be a little bit of ice mixed in. The rate at which heat will spread through such a medium will be governed by the process of thermal conduction. The equations of thermal conduction predict that the time a temperature rise needs to travel a given distance through a medium is proportional to the square of the distance. Based upon dry terrestrial soils, a reasonable estimate for this rate on Mars wouldbe about 16 square meters per year. We also need to estimate ho
w much gas is in the regolith. If you take zeolite minerals down to Martian temperatures and expose them to carbon dioxide, they will absorb enough carbon dioxide to comprise 20 percent of their solid weight. Martian regolith is not made of zeolite, but probably includes a lot of clay-like minerals, which are not too dissimilar. As a rough guess, let’s say that Martian regolith is saturated with about 5 percent carbon dioxide, and that the loose material has an average density of 2.5 tonnes per cubic meter. If that were the case, you would have to force out carbon dioxide held in regolith—outgas it—down to a depth of 200 meters to produce a 1,000-millibar (1 bar, Earth sea-level) pressure on Mars. Let’s say we induced a sustained artificial temperature rise at the surface of 10° Kelvin, good enough to outgas a significant fraction of what is in the regolith. This temperature rise would then travel down into the ground. The rate at which this would occur is shown in
Table 9.1
.
It can be seen that while it takes a very long time to reach significant depths, modest depths can be reached rather quickly. So, while it might take thousands of years to penetrate 200 meters to get a full 1,000 millibars out of the regolith, the first 100 millibars can be gotten out in just a few decades.
Once significant regions of Mars rise above the freezing point of water on at least a seasonal basis, the large amounts of water frozen into the regolith as permafrost would begin to melt, and eventually flow out into the dry riverbeds of Mars. Water vapor is also a very effective greenhouse gas, and since the vapor pressure of water on Mars would rise enormously under such circumstances, the reappearance of liquid water on the Martian surface would add to the avalanche of self-accelerating effects all contributing towards the rapid warming of the planet. The seasonal availability of liquid water is also the key factor in allowing the establishment of natural ecosystems on the surface of Mars.
The dynamics of the regolith gas-release process are only approximately understood, and the total available reserves of carbon dioxide won’t be known until human explorers journey to Mars to make a detailed assessment, so these results must be regarded as approximate and uncertain. Nevertheless, it is clear that the positive feedback generated by the Martian carbon dioxide greenhouse system greatly reduces the amount of engineering effort that would otherwise be re
quired to transform the Red Planet. In fact, since the amount of a greenhouse gas needed to heat a planet is roughly proportional to the square of the temperature change required, driving Mars into a runaway greenhouse with an artificial 10° Kelvin temperature rise only requires about 4 percent of the engineering effort that would be needed if the entire 50° Kelvin rise needed to raise the Martian tropics above the freezing point of water had to be engineered by brute force. The question we shall now examine is how such a 10° Kelvin global temperature rise could be induced.
TABLE 9.1
Rate of Outgassing of Atmosphere from the Martian Regolith
METHODS OF ACCOMPLISHING GLOBAL WARMING ON MARS
The three most promising options for inducing the required temperature rise to produce a runaway
greenhouse on Mars appear to be the use of orbital mirrors to change the heat balance of the south polar cap (thereby causing its carbon dioxide reservoir to vaporize); the mass production of artificial halocarbon (0;CFC”) gases in industrial facilities on the Martian surface; and the creation of widespread bacterial ecosystems capable of warming the planet through emission of large amounts of strong natural greenhouse gases such as ammonia and methane. We’ll look at each of these in turn. It may be, however, that the synergistic combination of several such methods may yield better results than any one of them used alone.
40
Orbiting Mirrors
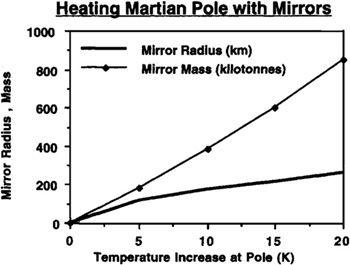
While the production of a space-based mirror capable of warming the entire surface of Mars to terrestrial temperatures is theoretically possible, the engineering challenges involved in such a task place such a project well outside the technological horizon of this book. A much more practical idea would be to construct a more modest mirror capable of warming a limited area of Mars by a few degrees. As shown by the data in
Figure 9.1
, a 4° Kelvin temperature rise imposed at the pole should be sufficient to cause the evaporation of the carbon dioxide reservoir in the south polar cap. Based upon the total amount of solar energy required to raise the temperature of a given area a certain number of degrees above the polar value of 150° Kelvin, it turns out that a space-based mirror with a radius of 125 kilometers could reflect enough sunlight to raise the entire area south of 70° south latitude by 5° Kelvin—more than enough. If made of solar sail-type aluminized mylar material with a density of 4 tonnes per square kilometer (about 4 microns thick), such a sail would have a mass of 200,000 tonnes. Many ships of this size are currently sailing the Earth’s oceans. Thus, while this is too large to consider launching from Earth, if space-based manufacturing techniques are available, its construction in space out of asteroidal or Martian moon material is a serious option. The total amount of energy required to process the materials for such a reflector would be about 120 MWe-years, which could be readily provided by a set of 5 MWe nuclear reactors such as might be used in piloted nuclear electric propulsion (NEP) spacecraft. Interestingly, if stationed near Mars, such a device would not have to orbit the planet. Rather,
solar light pressure could be made to balance the planet’s gravity, allowing the mirror to hover as a “statite” with its power output trained constantly at the polar region.
41
For the sail density assumed, the required operating altitude would be 214,000 kilometers. The statite reflector concept and the required mirror size to produce a g
iven polar temperature rise are shown in
Figures 9.8
and
9.9
.
If the value of T
d
is lower than 20° Kelvin, then the release of the polar carbon dioxide reserves by themselves could be enough to trigger the release of the regolith’s reserves in a runaway greenhouse effect. If, however, as seems probable, T
d
is greater than 20°K, then the addition of strong greenhouse gases to the atmosphere will be required to force a global temperature rise sufficient to create a tangible atmospheric pressure on Mars.
Producing Halocarbons on Mars
The most obvious way to increase the temperature on Mars is simply to set up factories there to produce the strongest greenhouse gases known to man, the halocarbons or CFCs that many consider to be currently threatening the Earth with a dangerous greenhouse effect, and then release them into the atmosphere. Here on Earth CFCs are also blamed for the destruction of the ozone layer. However, if we choose our halocarbon geenhouse gases carefully and employ varieties lacking in chlorine, we can actually build up an ultraviolet-shielding ozone layer in the Martian atmosphere. One good candidate for such a gas would be perfluoromethane, CF
4
, which also has the desirable feature of being very long-lived (stable for more than 10,000 years) in an upper atmosphere. In
Table 9.2 we show the amount of halocarbon gases needed in Mars’ atmosphere to create a given temperature rise, and the power that would be needed on the Martian surface to produce the required CFCs over a period of twenty years. If the gases have an atmospheric lifetime of one hundred years, then approximately one-fifth the power levels shown in the table will be needed to maintain the CFC concentration after it has been built up. The industrial effort associated with such a power level would be substantial, producing about a trainload of refined material every day and requiring the support of several thousand workers on the Martian surface. Power levels of about 5,000 MWe might be needed, which is about as much power as is used today by a large American city, such as Chicago. A total project budget of several hundred billion dollars might well be required. Nevertheless, all things considered, such an operation is hardly likely to be beyond the capabilities of the mid twenty-first century.
FIGURE 9.8
Solar sails of 4 tonnes/km
2
density can be held stationary above Mars by light pressure at an altitude of 214,000 km. Wasting a small amount of light allows shadowing to be avoided
.
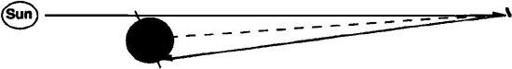
FIGURE 9.9
Solar sail mirrors with radii on the order of 100 km and masses of 200,000 tonnes can produce the 5°K temperature rise required to vaporize the CO
2
in Mars’ south polar cap. It may he possible to construct such mirrors in space
.
The Biological Solution
The level of effort required by humans to greenhouse Mars could be reduced substantially, however, if biologic
al assistants can be employed. This approach to terraforming has been championed by Carl Sagan ever since the 1960s, when he initiated the field of scientific terraforming speculation by suggesting that Venus might be made more habitable by seeding its atmosphere with algae that would consume the carbon dioxide in its atmosphere, thereby diminishing that planet’s hellish greenhouse effect.
42
That idea probably will never work, but in more recent Mars studies, Sagan and his collaborator James Pollack have pointed out th
at bacte
ria exist that can metabolize nitrogen and water to produce ammonia.
43
In addition to its minority presence in the atmosphere, nitrogen can likely be found on Mars in substantial amounts in regolith nitrate beds. Other bacteria can synthesize water and carbon dioxide into methane. Now, while not as good as halocarbons, both ammonia and methane are excellent greenhouse gases, thousands of times more powerful on a molecule-for-molecule basis than carbon dioxide. If an initial greenhouse condition were to be created by polar mirrors or CFC manufacture, thereby putting some liquid water into circulation, it may be possible that a bacterial ecology could be set up on the planet’s surface that would accelerate the process by producing large amounts of ammonia and methane. In fact, if 1 percent of the planet’s surface were to be covered by such bacteria, and we assume that they operate at about 0.1 percent efficiency in converting the energy of sunlight into chemical compounds, then around one billion tonnes of methane and ammonia would be produced each year. This is enough to warm the planet 10° Kelvin in about thirty years.