The Cerebellum: Brain for an Implicit Self (21 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

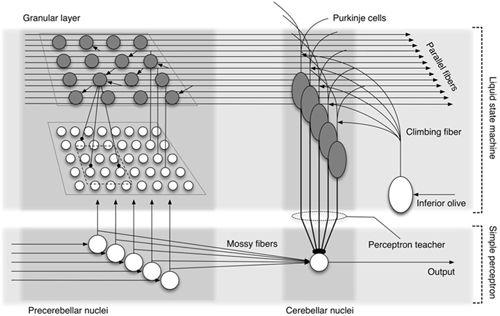
To model the cortical and nuclear dual memory mechanism in the cerebellum, Yamazaki and Nagao (
2008
) proposed a hybrid model that used a liquid state machine for the cerebellar cortex and a simple perceptron for vestibular and cerebellar nuclei (
Figure 25
). The authors assumed that teacher signals for the simple perceptron were derived from Purkinje cell output as in the Medina and Mauk’s (
1999
) model, but with different cellular processes. The hybrid model enabled the computer simulation of short- and long-term gain adaptation of the OKR.
Figure 25. Hypothetical hybrid organization of a cerebellar circuit.
This model involves a combination of the cerebellar cortex as a liquid state machine (
Figure 24
) and the precerebellar-cerebellar nuclei as a simple perceptron (
Figure 5
). Mossy fiber-cerebellar nuclear cell synapses are considered to undergo plastic changes using teacher signals fed by Purkinje cells to the cerebellar nuclei. (Courtesy of Tadashi Yamazaki.)
Oscarsson (
1979
) delineated a narrow longitudinal zone as a structural-functional unit of the cerebellar cortex and called it a “microzone.” Today, we consider the microzone concept to also pertain to other small groups of neurons, for example, cerebellar and vestibular nuclear neurons as recipients of Purkinje cell inhibition and IO neurons as the source of climbing fibers (
Figure 26
). A small group of neurons of the parvocellular red nucleus that project to the neocerebellum via the IO may also be attached to a microzone (see also Color Plate V). These structures conjointly constitute what can be termed a “cortico-nuclear microcomplex” and shortened to microcomplex (
Ito, 1984
). The presence of such a microcomplex has been demonstrated in various regions of the cerebellum. In the intermediate part of the cerebellar hemisphere, a microcomplex has been visualized by a combination of zebrin immunohistochemical and tracer techniques (
Pijpers et al., 2005
). Injections of small amounts of biotinylated dextran amine into either the medial or dorsal accessory olives label strips of climbing fibers and also patches of climbing fiber collaterals in the interpositus nucleus. On the other hand, gold-lectin injection into selected parts of the interpositus nuclei retrogradely labels both Purkinje cells and
in vivo
neurons. On the basis of the cellular data reviewed in
Chapters 4
–
6
, microcomplexes seem to function as follows. They transform mossy fiber inputs to nuclear neuron outputs and are equipped with an
in vivo
input for instruction on error learning via climbing fibers. They also have neuromodulatory inputs for switching the behavioral expression of their neuronal circuits. Assuming that a microzone occupies an area of ~0.2 × 50 millimeters squared (
Andersson and Oscarsson, 1978
), the human cerebellum, which is 50,000 millimeters squared in area, is composed of ~5,000 microcomplexes. Each of these complexes is presumed to be incorporated into a functional system composed of the spinal cord, brainstem, and cerebral cortex (
Ito, 1984
).
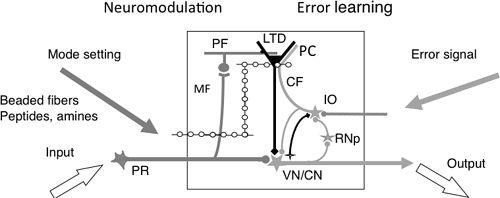
Figure 26. A cerebellar microcomplex functioning as an embedded computing system.
A, This schematic details the major components of a cerebellar microcomplex. From left to right, these components and their role include the following: Input, via precerebellar neurons; Mode setting, beaded fibers release a monoamine or a peptide as a neuromodulator (e.g., serotonin, orexin) to modify the mode of operation of the circuits of the microcomplex; MF, mossy fibers serve as a major input to the cerebellar cortical circuit; LTD, long-term depression serves as a memory process; CF, climbing fibers convey an error signal; IO, the inferior olive serves as a teacher; Error signal, informs about errors in the performance of the entire system, including the microcomplex; Output, signals from nuclear neurons that are sent from the cerebellum to postcerebellar neurons; VN/CN, vestibular and cerebellar nuclear neurons.
Mapping of the flocculus effects in response to various electrical microstimulations revealed microzones associated with each component of the VOR (horizontal, vertical, and rotatory) (
Nagao et al., 1985
), an eye blink and neck muscle contraction (
Nagao et al., 1984
), and a rise in mean blood pressure (
Nisimaru et al., 2010
) (
Figure 29
). The microcomplexes involved in the VOR have been analyzed with various techniques, as will be reviewed in
Chapter 10
, “
Ocular Reflexes
”; in particular that in the horizontal VOR has been labeled systematically by retrograde transneuronal tracing with the rabies virus from the medial rectus muscle in guinea pigs (
Graf et al., 2002
). In another study, the microcomplex structure in the C
3
zone of the anterior lobe of the cerebellum has been analyzed intensively in connection with the withdrawal reflex (
Ekerot et al., 1995
;
Garwicz et al., 1998
;
Jörntell and Ekerot, 2003
). The C
3
zone, which receives mossy fiber and climbing fiber inputs from receptive fields in the forelimb skin, contains 30–40 longitudinal microzones lying side by side, each 50–150 micrometers wide. Purkinje cells and inhibitory interneurons in each microzone receive climbing fiber inputs from the same cutaneous receptive field. Climbing fibers in adjacent microzones are activated from adjacent skin areas, forming a detailed somatotopic map of the ipsilateral forelimb’s skin, particularly its distal parts. Adjacent microzones innervate, in turn, adjacent cell groups in the anterior interpositus nucleus. Through further projections to the red nucleus, these microzones control movement components that have specific relationships with the location of climbing fiber receptive fields (see
Chapter 11
).
Computational modeling has been fruitful in reproducing theoretical principles conceived for complex neuronal networks. Bottom-up, experiment-based realistic simulation has also been of value. Unique models of the cerebellum have thus been developed. Given the complex diverse features of the cerebellum, however, further advanced models are clearly needed. There is also a possibility that hardware models of the cerebellum can be developed using silicon analogues of neurons (
Rachmuth and Poon, 2008
).
An eyeball is often likened to a single-joint limb, with three degrees of freedom for movement (horizontal, vertical, rotatory). For securing stable accurate vision, however, eyeballs are equipped with four major reflexes. These are evoked by vestibular and visual afferent signals, and they compensate for movements of the head and its environment. In this chapter we review current knowledge about these ocular reflexes and their cerebellar control. The four reflexes provide relatively simple control system models of cerebellar adaptation and compensation, and they have helped considerably in the design of robust experimental paradigms for testing several aspects of the function of the cerebellum.
The VOR is mediated by a tri-neuronal arc consisting of vestibular afferents, VOR relay neurons, and motoneurons supplying extraocular muscles. To control eye movement and eye position in three-dimensional space, the input VOR afferents are those that originate in the three semicircular canals (horizontal, anterior, posterior) and the two otolith organs (utricle, saccule) in each labyrinth (
Uchino et al., 1996
;
Isu et al., 2000
). The output of VOR motoneurons acts on six extraocular muscles (medial/lateral rectus, superior/inferior rectus, superior/inferior oblique) for each eye (
Figure 27
). Under experimental conditions, yaw, pitch, and roll of the head induce, respectively, the horizontal, vertical, and rotatory components of the VOR. Utriculo- and sacculo-ocular reflexes are evoked by linear motion and static tilt of the head. Under freely moving conditions, the VOR is mediated by the concerted operation of the parallel pathways that link the ten sensors in two labyrinths to the twelve muscles in the two eyes (
Ezure and Graf, 1984b
).
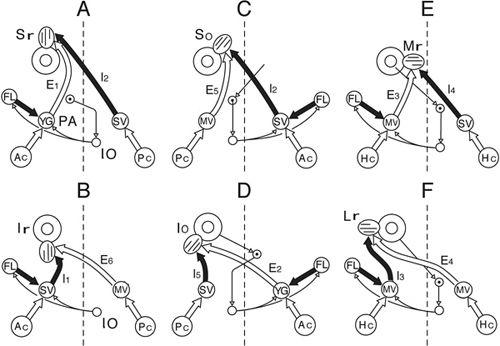
Figure 27. Canal specific pathways of vestibuloocular reflexes.
The A–F drawings show VOR pathways converging onto six different extraocular muscles including A, Sr (superior rectus); B, Ir (inferior rectus); C, So (superior oblique); D, Io (inferior oblique); E, Mr (medial rectus); and F, Lr (lateral rectus). Other abbreviations: Ac, anterior canal; E
1-6
/I
1-6
, secondary vestibular neurons (E, excitatory; I, inhibitory); FL, flocculus; Hc, horizontal canal; MV, medial vestibular nucleus; Pc, posterior canal; SV, superior vestibular nucleus. (From
Ito et al., 1977
.)
Among the component pathways of the VOR, those functioning in the horizontal VOR have been investigated the most extensively. Hence, unless otherwise indicated, the VOR discussed in this chapter is for its horizontal component. The horizontal semicircular canals are stimulated by ipsilateral head rotations, and they send their neural signals via the primary vestibular nerve fibers to VOR relay cells located in the vestibular nuclei. These cells send, in turn, either excitatory or inhibitory signals to the lateral/medial rectus motoneurons located in the abducens/oculomotor nuclei. The excitatory transmitter for the primary vestibular nerve is glutamate (
Raymond et al., 1984
), and those for the secondary VOR relay neurons are glutamate and aspartate (
Kevetter and Hoffman, 1991
). The inhibitory transmitter for the secondary VOR relay neurons is glycine (
Spencer et al., 1989
). When the head is rotated to one side, contraction of the ipsilateral medial rectus and contralateral lateral rectus muscles and relaxation of the ipsilateral lateral rectus and contralateral medial rectus muscles cause both eyes to rotate in the direction opposite to that of head rotation.
Velocity storage.
Head rotation in darkness at a constant velocity induces a nystagmus that consists of alternating slow phases representing the VOR and quick phases that reset eye position. In this situation, impulses evoked in primary vestibular afferents attenuate rapidly within 5 seconds. In parallel, however, the slow-phase velocity of nystagmus decays slowly with a time constant of ~20 seconds. To explain this discrepancy, the VOR arc has been assumed to involve a velocity storage mechanism. It might store the initial head velocity as transduced by the semicircular canals and maintain it despite decay in the firing rate of the canal afferents (
Raphan et al., 1979
). Because electrical stimulation of the nodulus and uvula induces a rapid decrease in horizontal slow-phase velocity, these cerebellar regions might control velocity storage (
Solomon and Cohen, 1994
) in contrast to the control of VOR gain by the flocculus.