Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience
The Cerebellum: Brain for an Implicit Self (22 page)

Neural integrator.
In vestibular nuclei and motoneurons for extraocular muscles, the head velocity signals generated by the labyrinth are converted to eye-velocity and eye-position signals. Hence, the VOR arc must be equipped with a neural integrator (Cannon and Robinson, 1985), which, for the horizontal VOR, may involve the nucleus prepositus hypoglossi and/or commissural inhibitory projections between bilateral vestibular nuclei (
Arnold and Robinson, 1997
). The integrator for the vertical VOR is provided by the interstitial nucleus of Cajal in the midbrain (
Fukushima et al., 1992
). Velocity storage and neural integrator action may represent functions of the same neuronal circuit, but this has yet to be clarified.
Figure 28
illustrates the wiring diagram of the VOR pathway and its connection with the flocculus, fitted to a framework of an adaptive control system. Vestibular nuclear neurons relaying the VOR arc placed as the controller, whereas motoneurons, extraocular muscles, and eyeballs are taken together as the controlled object. We found early on that Purkinje cells in the flocculus directly inhibited relay neurons of the VOR (
Fukuda et al., 1972
;
Kawaguchi, 1985
). This led to the proposal of the flocculus hypothesis—that is, that this structure controls adaptively the VOR (
Ito, 1982
). One aspect of this hypothesis was that Purkinje cell inhibition was exerted on one of the two VOR arcs converging onto each extraocular muscle (
Figure 27
;
Ito et al., 1977
). These Purkinje cells were shown to form VOR-specific microzones in the flocculus, as mapped in
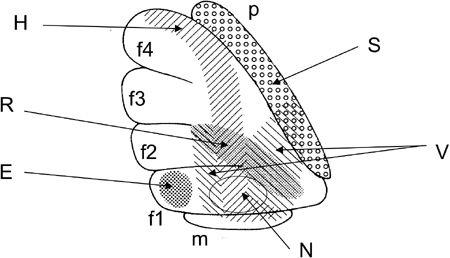
Figure 29
. Microzones have now been demonstrated in various species (rabbits,
Nagao et al., 1984
;
Van der Steen et al., 1994
;
De Zeeuw et al., 2004
; cats,
Sato et al., 1983
,
Sato and Kawasaki, 1984
; guinea pigs,
Graf et al., 2002
; rats, Billig and Balaban, 2005; mice,
Schonewille et al., 2006
). Retrograde transneuronal tracing using the rabies virus from the left medial rectus muscle demonstrated vividly the H-zone in the flocculus (
Graf et al., 2002
).
Figure 28. Wiring diagram for cerebellar control of the horizontal vestibular ocular reflex.
This drawing shows the tri-neuronal arc of the horizontal VOR (broken line rectangle cs) and the flocculus superimposed upon it. The broken line rectangle mc encloses eVN and iVN (excitatory and inhibitory vestibular nuclear neurons) as a controller and the flocculus which provides its adaptive mechanism). The box on the right encloses the controlled object component. Other abbreviations: AOS, accessory optic system; CF, climbing fibers; IO, inferior olive; LR, lateral rectus muscle; MF, mossy fibers; Mr, medial rectus muscle; PC, Purkinje cells; VA, vestibular afferents. Symbols: filled circles, excitatory connections; square, inhibitory connections. Note that the broken line rectangle contains the microcomplex, which acts as an adaptive controller.
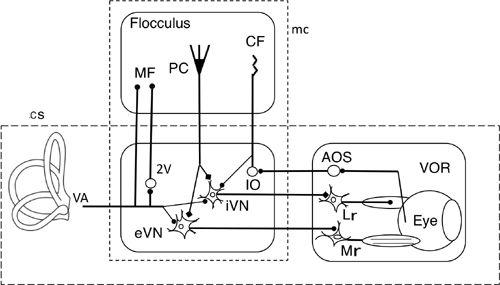
Figure 29. Microzones mapped in the fllocculus.
Lateral surface of a rabbit flocculus on the left side. Stimulation through a glass pipette electrode evoked site-dependent effects from the shaded or dotted areas: horizontal (H), vertical (V), or rotatory (R) eye movement, eye blink (B), contraction of neck muscles (N), or a transient increase in the mean blood pressure (S). Based on Nagao et al. (
1984
,
1985
) and Nisimaru et al. (
2010
).
The marked adaptation in horizontal VOR gain was demonstrated when the vestibular-visual relationship was changed using Dove prism goggles or magnifying lenses, or by exposing an animal to an in-phase/out-of-phase combination of horizontal turntable movements and screen rotation (
Chapter 3
, “
The Cerebellum as a Neuronal Machine
”). Adaptation has also been demonstrated to occur in the vertical VOR when an animal was fixed to a chair with the animal’s right or left side down and rotated horizontally in the center of a horizontally rotating screen (squirrel monkeys;
Hirata and Highstein, 2001
). In another study, cross-axis adaptation occurred in the cat’s horizontal VOR when horizontal turntable rotations (yaws) were combined with vertical optokinetic motions. This resulted in the VOR (measured in the dark) acquiring a vertical component. Also, the sensitivity of vestibular
nuclear neurons increased significantly to yaw in the dark, but not to vertical plane rotations (
Quinn et al., 1996
). Similarly, cross-axis adaptation in the monkey was shown by having the animal track a vertically moving target spot synchronized with horizontal whole body rotation. Before training, the horizontal trapezoidal rotation resulted in a collinear VOR with a mean latency of 15 ms. After training, the collinear VOR remained unchanged but an orthogonal, cross-axis VOR developed. It had a mean latency of 42 milliseconds with a gain (eye velocity/chair velocity) of 0.2, which decayed with a mean time constant of 80 milliseconds (
Sato et al., 1999
).
Pharmacological or genetic methods have been used to block conjunctive LTD in order to test its potential role in horizontal VOR adaptation. For example, such adaptation was abolished in rabbits and monkeys when their flocculus was superfused with hemoglobin, which blocks conjunctive LTD induction by absorbing NO (
Nagao and Ito, 1991
). VOR adaptation was also blocked in mutant mice null-deficient in Purkinje-cell-specific PKG-1 (
Feil et al., 2003
) and in transgenic mice overexpressing in Purkinje cells the pseudo-substrate protein kinase C inhibitor (
De Zeeuw et al., 1998
). Conjunctive LTD was absent in cerebellar slices derived from these two types of mice. The signaling effects of flocculus Purkinje cells, which underlie VOR adaptation, have been analyzed as described in the following text.
Vestibular mossy fiber input.
The flocculus has long been known to be that part of the vestibulocerebellum that receives the projection of primary vestibular afferents in the form of mossy fibers (
Brodal, 1972
). This notion, however, was reexamined subsequently in various species. In rabbits, the presence of primary vestibular afferents to the flocculus was negated (
Gerrits et al., 1989
). Later, however, it was shown to engage 2% of the primary vestibular afferents, whereas 64%–89% of these afferents were shown to project to the nodulus and uvula (Barmack et al., 1993). In cats, the primary vestibular projection to the flocculus was described as being substantial but less pronounced than that to the nodulus and uvula (
Korte and Mugnaini, 1979
). In monkeys, the same projection to the flocculus was measured to be 6% (Nagao et al., 1997a), and in rats the value was 10% (Osanai et al., 2000). This relative paucity of primary vestibular afferents to the flocculus, however, could be supplemented by secondary projections from vestibular nuclei (Barmack, 2003). Head velocity-sensitive neurons have indeed been found in vestibular nuclei (
Scudder and Fuchs, 1992
). Some mossy fiber terminals in the monkey flocculus and ventral paraflocculus were shown to respond primarily to vestibular stimuli (
Lisberger and Fuchs, 1978
;
Noda and Warabi, 1987
;
Markert et al., 1988
). These observations support the idea that primary and/or secondary mossy fiber inputs convey vestibular signals to the flocculus.
Climbing fiber input.
A prominent pathway arises from the retina and reaches the flocculus in the form of climbing fibers (
Maekawa and Simpson, 1973
). This pathway is mediated by the nucleus of the optic tract and the accessory optic system (
Simpson, 1984
). The functional role of this pathway has been assumed to convey retinal slips as sensory errors to the flocculus. However, this idea was confounded by the finding that flocculus Purkinje cells often modulate climbing fiber discharges during a VOR in the dark (
Ghelarducci et al., 1975
;
Belton et al., 2002
;
Simpson et al., 2002
). This implies the involvement of vestibular/oculomotor components in the signals conveyed by the climbing fiber pathway. Normally, experiments on the VOR are complicated by the difficulty in isolating motor signals from sensory signals because the latter cause movements that generate motor signals. In recent studies, efforts have been made to break the normally tight relationship between instantaneous retinal slip and eye movement. The results of such experiments will be discussed in
Chapter 12
, “
Adaptive Control System Models
,” in relation to the general question of whether climbing fibers represent sensory or motor signals. Here, one may ask about the biological role of the climbing fiber discharges during a VOR in the dark. A relevant observation was that if rotation in darkness occurred immediately followed learning, the gain of the VOR reverted toward its
prelearning value, thereby indicating that expression of the memory was disrupted. If, after gain-down learning, the cat spent another 60 minutes stationary without form vision (the cat was stationary either in the light with the lenses covered by filter paper or in complete darkness), subsequent disruption did not occur. This suggested that the memory had been consolidated (
Titley et al., 2007
).
Eye-movement-related signals.
In addition to the previously mentioned simple-spike discharges in response to vestibular mossy fiber inputs, Purkinje cells in the flocculus also exhibit simple-spike discharges representing either eye acceleration, eye velocity, or eye position (
Mizukoshi et al., 2000
;
Omata et al., 2000
;
Hirata and Highstein, 2001
;
Blazquez et al., 2003
). The possibility has been entertained that such eye-movement signals are in response to proprioceptive feedback from extraocular muscles. This was supported only in part because the blockade of muscle afferents by local anesthesia was shown to reduce simple-spike discharges related to eye velocity by only 31% (rabbits:
Miyashita, 1984
). This finding suggested that the remaining two-thirds of such discharge must have an intrinsic origin. This issue is still open, with the following three possibilities on the forefront of consideration.
First, the source of such an intrinsic mechanism might be eye-velocity- and eye-position-sensitive neurons located in vestibular nuclei (
Scudder and Fuchs, 1992
). These neurons might convert head velocity signals to eye-velocity signals. Additionally, eye position signals might be generated from eye-velocity signals by means of a neural integrator, as defined above. It was also reported that some mossy fibers in the flocculus/ventral paraflocculus respond to eye movements induced by a saccade, head rotation, or smooth tracking of a moving target (
Lisberger and Fuchs, 1978
;
Noda and Warabi, 1987
). Hence, one hypothesis is that VOR relay neurons convert head velocity signals to eye-velocity signals and send them to the flocculus/ventral-paraflocculus via recurrent axon collaterals (
Miles and Lisberger, 1981
).
The second possibility is that the simple spikes of Purkinje cells might express eye-movement-related signals received via mossy fibers from sources other than VOR relay neurons. For example, midline paramedian tract neurons were reported to send vertical eye-velocity signals to the flocculus via mossy fibers (
Nakamagoe et al., 2000
). It remains unknown, however, if other paramedian tract neurons mediate horizontal eye-velocity signals.
The third possibility is that eye-movement-related signals are generated in the flocculus (
Ito, 1998
). Indeed, simple-spike activity in the flocculus changed rapidly preceding the eye-velocity change that occurred in response to a change in the direction of the visual surroundings. These rapid responses should represent
sensory signals from which the eye-movement-related simple-spike activity was generated (cat;
Mizukoshi et al., 2000
). Such a conversion might be secured by error learning as driven by the climbing fibers’ retinal error signals. The latter contain eye-movement-related components because retinal errors represent a discrepancy of movements between the eyes and their visual surroundings relative to the head. This possibility is supported by observations about the OFR, as described later. In brief, simple-spike discharges in ventral paraflocculus Purkinje cells during the OFR are modulated to become a mirror image of the temporal profile of climbing fiber signals. This suggests that during the OFR, climbing fiber signals refine simple-spike activity, possibly by inducing conjunctive LTD (
Shidara et al., 1993
).
