Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience
The Cerebellum: Brain for an Implicit Self (33 page)

Purkinje cells whose simple-spike discharge rates were modulated in close correlation with saccadic eye movements were found in fairly restricted areas in the cerebellar hemisphere, mostly in crus II with some in the deep folia of crus I. One study showed that two-thirds of saccade-related Purkinje cells began to change their simple-spike discharge rate 20–100 milliseconds prior to the onset of saccades. The remaining one-third changed their activity at approximately the time of saccade onset. These saccade-related Purkinje cells showed no changes in their activity during smooth-pursuit eye movements (
Mano et al., 1991
). Thus, a group of Purkinje cells in crus I and II of the cerebellar hemisphere must play a role in the control of voluntary saccadic eye movements.
Vergence refers to eye movements that rotate the eyes simultaneously in opposite directions (disconjugate eye movements). In frontal-eyed human and nonhuman primates, whereas the smooth-pursuit system moves both eyes in the same direction to track movements in the frontal plane (frontal pursuit), the vergence system moves the left and right eyes in the opposite direction to track targets moving toward or away from the observer (vergence tracking). Information related to vergence eye movements, namely retinal disparity and the blur signals that elicit them, is coded independently of signals related to frontal pursuit. Fukushima et al. (
2002
) demonstrated that frontal eye field neurons modulate strongly during both frontal pursuit and vergence tracking, thereby suggesting that they play a role in the control of three-dimensional eye movements. These neocortical neurons may
function as part of a system that enables primates to track and manipulate objects moving in a three-dimensional space.
Recently, vergence-related neurons have been recorded in the posterior vermis of monkeys (
Nitta et al., 2008
). Also, vermal lesions have been shown to impair vergence (
Takagi et al., 2003
). However, there may well be multiple areas of the cerebellum involved in the control of vergence, as is clearly the case for smooth pursuit.
In addition to the frontal eye field located in the dorsolateral frontal cortex, another eye-movement-related area resides within the dorsomedial frontal cortex (DMFC). As reviewed by Tehovnik et al. (
2000
), the DMFC differs from the frontal eye field in several respects. It contains neurons that contribute to the control of both limb and eye movements, whereas the frontal eye field is dedicated to the execution of saccadic and smooth-pursuit eye movements. A study showed that saccades evoked by electrical stimulation of most sites within the DMFC tended to shift the center of gaze to a particular location within craniotopic (head-centered) space, whereas the frontal eye field had a retinotopic (eye-centered) code for saccades (
Schlag and Schlag, 1987
). The DMFC contains a somatotopic map with the eyes represented rostrally and the hindlimbs caudally. This is in further contrast to the frontal eye field, which has no such map. Furthermore, lesions of the DMFC have a minimal effect on the production of saccadic eye movements and no effect on the execution of smooth-pursuit movements. Imaging in humans and single-unit recording in monkeys have suggested that the DMFC is involved in various motor tasks unrelated to eye movements. Taken together, whereas the frontal eye field acts as the controller of oculomotor neuronal circuits for voluntary saccadic and smooth-pursuit eye movements, the DMFC seems to expand on the functions of the frontal eye field by integrating them with non-oculomotor cortical functions.
The DMFC consists of subareas, the supplementary eye field (medial eye field), and the presupplementary eye field. Whereas the frontal eye field has a prominent role in prosaccade initiation, the supplementary eye field is required for antisaccade tasks (
Everling and Fischer, 1998
). In this task, the subject was instructed to look not at the location of a flashed stimulus as the cue but in the opposite direction, at an equal distance from the central fixation point. The subject was also instructed to glance at the cue. This ability of antisaccade was lost in patients with lesions in the frontal lobe (
Guitton et al., 1985
). Neuronal discharges in the supplementary eye field were found to be consistently greater before antisaccades than before prosaccades with the same trajectories (
Schlag-Rey et al., 1997
). How the brain computes an inverted vector for antisaccades is still an open question.
The frontal eye field acts as the cortical controller for smooth pursuit, saccades, and vergence. For saccades, the brainstem saccade generator circuits involving the superior colliculus and A-zone-fastigial nucleus microcomplex provide the controlled object (
Figure 40
in
Chapter 12
, “
Adaptive Control System Models
”). Yet to be identified are the neuronal circuits that provide the analogous controlled objects for smooth pursuit and vergence. Although both the frontal eye field and crus I and crus II of the cerebellar hemisphere are involved in smooth pursuit and saccades, there is no anatomical evidence for a loop connection involving these areas such as those demonstrated in other prefrontal areas (for further discussions, see
Chapter 15
,
Section 4
).
After reviewing the data on voluntary movements of the arms, hands, fingers, and eyes in
Chapters 13
and
14
, we are now ready to address control system models of voluntary movements that have helped define the mechanisms and roles of the cerebellum in the control of voluntary movements.
Chapter 1
, “
Neuronal Circuitry: The Key to Unlocking the Brain
,” emphasized that two forms of internal models (forward and inverse) are essential components of the overall control system for such movements. Both address the role of learning in acquiring skilled movements. Internal models also explain how sensory cancellation occurs during learning to obviate the effects of unwanted sensory perturbations, these being inevitable when beginning new voluntary tasks, in general, and those requiring considerable skill, in particular.
In performing voluntary movements such as pointing and reaching (
Chapter 13
, “
Voluntary Motor Control
”), the primary motor cortex acts as a controller. It follows instructions received from its higher centers (e.g., supplementary motor cortex, premotor cortex, anterior cingulate gyrus) and, in turn, drives a controlled object composed of lower motor centers in the brainstem and spinal cord and a motor apparatus in the periphery. The primary motor cortex receives somesthetic signals via peripheral nerves as feedback (Asanuma et al., 1979). In addition, the primary motor cortex is incorporated into a unique structure of the brain; the cerebrocerebellar communication loop that functions as an internal forward model.
A typical cerebrocerebellar communication loop is formed between the primary motor cortex and the C
1
/C
3
zones of the intermediate part of the cerebellar hemisphere. These two areas are linked via the anterior interpositus nucleus, the
ventrolateral thalamic nucleus, and the pontine nucleus/nucleus reticularis tegmenti pontis (
Figures 43
and
46
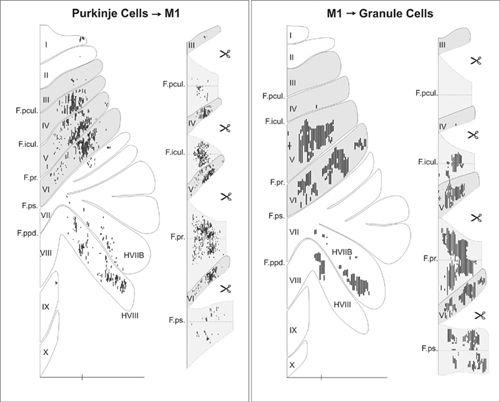
). This cerebrocerebellar communication loop has recently been remapped using the transneuronal transport of neurotropic viruses. It has been shown that neurons in the arm area of the primary motor cortex receive inputs from Purkinje cells located primarily in lobules IV–VI of the C
1
and C
3
zones. In turn, neurons in the arm area of the primary motor cortex project back to the equivalent arm sites in the cerebellum, primarily via the pontine nucleus (
Kelly and Strick, 2003
) (
Figure 42
).
Figure 42. Input-output organization of cerebellar loops with the primary motor cortex.
(Left) The distribution of Purkinje cells (small dots) that project to the arm area of the primary motor cortex. These neurons were labeled after retrograde transneuronal transport of rabies virus from injections into the arm area of the primary motor cortex. (Right) The distribution of granule cells (fine lines) that receive input from the arm area of the primary motor cortex. These neurons were labeled after anterograde transneuronal transport of the H129 strain of HSV1 from injections into the arm area of the primary motor cortex. The shaded areas on the flattened surface maps (diagrams on the left side of each panel) are unfolded on the right side of each panel to show the distribution of labeled neurons in the relevant cerebellar cortical fissures. The small icons of scissors in the diagram indicate places where the maps have been cut to facilitate the unfolding process. Scale bars, 15 millimeters. (From
Kelly and Strick, 2003
.)
The various roles of cerebrocerebellar loops have been considered. If it represents an internal model simulating the external feedback from a controlled object and if learning makes the simulation exact, the primary motor cortex would be able to perform precise control by referring to the output of this internal model instead of the real controlled object. A model based on a design of a “Smith Predictor” suggested that an internal model replaces long and unavoidable feedback delays (
Miall et al., 1993
). In developing a computational theory for control of a robot’s arm Kawato et al. (
1987
) defined a “forward model” as mimicking the input-output relationship of a controlled object. This was in contrast to another inverse model that mimicked a reciprocal relationship of the forward model (see following description). When the microcomplex shown in
Figure 43
contains a forward model that simulates the kinematics of the controlled object, the primary motor cortex should be able to perform a precise movement using internal feedback from the forward model instead of external feedback from the real control object. Another closely related idea is that the cerebellum estimates the current state of the motor system. To this end, it may calculate a “state estimate” by combining sensory information about the last known position of the arm with predictions of its future responses to the most recent movement commands, and thereby accurately plan and control a reaching movement (
Miall et al., 2007
). This hypothesis was supported by the finding that TMS stimulation over the cerebellum caused errors in the later component of the trajectory of an arm reaching for a target (
Chapter 13
).
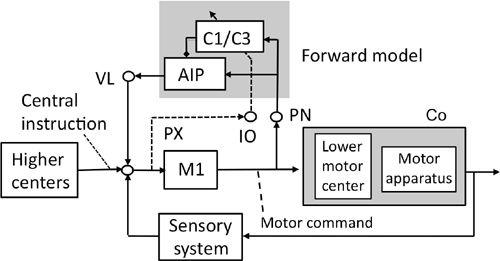
Figure 43. A forward-model-based control system scheme for voluntary movements.
See the text for further information about this figure. The shade in the upper middle encloses the microcomplex that acts as a forward model. The shade on the right encloses the controlled object. Abbreviations: AIP, anterior interpositus nucleus; C1/C3, two longitudinal zones in the intermediate part of the cerebellum; Co, controlled object; IO, inferior olive; M1, motor cortex; PN, pontine nucleus; PX, possible pathway to IO; VL ventrolateral thalamic nucleus. Symbol: dotted and slanted arrow from the IO’s climbing fiber pathway. (Based on
Ito, 2001
,
2006
.)
Learning a voluntary movement by repeated practice can be considered to be a process whereby a forward model is formed and reformed in the cerebellum through modification of the input-output relationship of the involved (relevant) microcomplex. A well-formed forward model would certainly be of use in acquiring motor skills. When we begin executing a novel voluntary movement, we initially require feedback to ensure a progressively improving performance. But when the quality of the movement reaches a certain level, we are then able to perform the task without feedback. For example, one can quickly learn to take a finger to the nose with the eyes closed. This capability is impaired in dysmetric patients with cerebellar damage (
Chapter 2
, “
Traditional Views of the Cerebellum
”). It suggests that a forward model in the cerebellum is required to accurately locate the nose relative to the finger in the absence of visual feedback. An interesting case was reported by Sasaki (
1985
) and discussed by Leiner et al. (
1987
). It involved a medical doctor who suffered from an infarction in the posterior lobe of the cerebellum on the left side. One day, after he had a short attack, which disturbed his consciousness for about several seconds, he tried the finger-nose test on himself. As his finger approached his nose (or eye, ear, or navel), he noticed that the target disappeared
from his mental image of his body. Instead, he imagined a space with a several-centimeter radius around the target to be a “sea of clouds.” As a result, he had to point his finger into the middle of this vague space. This conscious experience suggests that a forward model within the cerebellum represents a target to be reached in a mental image of the body’s various parts.
