Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience
The Cerebellum: Brain for an Implicit Self (35 page)

Kawato and his colleagues developed a new model with complex architecture: the “modular selection and identification for control (MOSAIC).” It was designed for motor learning and control as based on multiple pairs of forward (predictor) and inverse (controller) models. The MOSAIC learns simultaneously the multiple inverse models necessary for control as well as how to select a set of inverse
models that is appropriate for a given task. It combines feedforward and feedback sensorimotor information so that controllers can be selected both prior to a movement and subsequently during this movement. The MOSAIC can operate in a number of ways. For example, it can learn to manipulate multiple objects and switch appropriately between them. After such learning, the model can generalize novel objects whose dynamics are within the range of already learned ones. In this manner, the MOSAIC can learn the dynamics required for a given movement task and select the controller before executing the task (
Haruno et al., 2001
).
We now know that a microcomplex is combined with a control system in two distinct ways. One way is defined in
Chapter 12
,
Section 8
in terms of V- and C-types, in which a microcomplex is embedded in a control system in such a way that the nuclear component of the microcomplex serves both as a controller and as a part of an adaptive mechanism (
Figures 28
,
32
,
33
,
35
–
38
,
40
,
41
). The other way is that a microcomplex forms an internal model to assist the cortical controller (
Figures 43
,
44
). Note that in the latter cases the microcomplex is a separate unit from the controller, not part of it as in the former cases. How these two forms of microcomplexes switch as a function of the evolution of the cerebellum is an interesting question addressed in the following discussion.
As mentioned previously, the internal forward model was conceived in connection with the cerebrocerebellar communication loop as assisting the primary motor cortex in voluntary motor control (
Figure 43
). However, this form of internal model may be provided whenever there is a communication loop attached to a controller. For example, the rubrospinal tract is known to extrude axon collaterals that project to the lateral reticular nucleus (
Robinson et al., 1987
), which is a source of mossy fiber afferents to the cerebellum (
Chapter 6
). It is therefore possible that a rubrocerebellar communication loop is formed (
Figure 45
), and it may enable a forward-model-based control of a long-looped reflex mediated by the magnocellular red nucleus (such as grasping). A microcomplex involving the C
1
/C
3
zones and the anterior interpositus nucleus is also attached, possibly as an internal forward model, to the primary motor cortex (
Figure 46
). When we move further to the lateral cerebellar hemisphere, a microcomplex involving D
1
or D
2
zones and dentate nucleus forms the cerebrocerebellar communication loop (
Figure 49
,
Chapters 16
and
17
). It may thus appear that whereas the adaptive control form of microcomplexes (N- and C-types) prevails in the evolutionarily old cerebellum, it changes to the internal forward-model-based form located in the intermediate to the lateral cerebellar hemisphere.
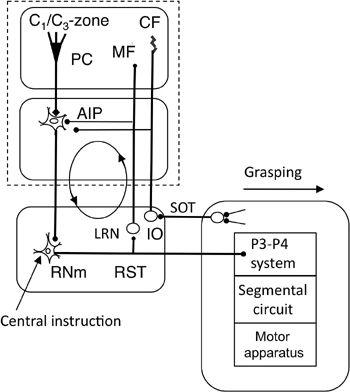
Figure 45. Forward-model-based control by the magnocellular red nucleus.
This wiring diagram focuses on the recurrent projection from the rubrospinal tract to the cerebellum via the lateral reticular nucleus (LRN), which may form a rubrocerebral communication loop as indicated by the circle with arrows. Other abbreviations: IO, inferior olive; RNm, magnocellular red nucleus: RST, rubrospinal tract; SOT, spinoolivary tract. Symbol: filled bulbs at the end of projection lines, excitatory synapses. Other abbreviations and symbols are as in
Figure 33
. Reflexive grasping might well be subserved by this system.
An interesting aspect of control system structures is that anterior interpositus neurons project branches of the same axons to both the magnocellular red nucleus and the primary motor cortex (the latter via the ventrolateral thalamic nucleus) (
Toyama et al., 1970
). With these projections, the same microcomplex involving the C
1
/C
3
zone and the anterior interpositus nucleus is linked to both the magnocellular red nucleus (
Figure 45
) and the primary motor cortex (
Figure 46
). How can the same microcomplex serve as a common forward model for two separate controllers? This is indeed possible because for both controllers, the microcomplex is to be tuned to simulate the common controlled object at segmental levels of the spinal cord. This is illustrated in
Figure 47
for the case of a feedforward model. Thus, a microcomplex tuned during reflex activity can also be effective during voluntary activity, and vice versa. Such a hybrid control system (
Figure 9C
) is worthy of testing because it answers the long-standing question of how the same anterior interpositus neurons can control two descending systems at the same time.
Figure 46. Forward-model-based control by the primary motor cortex.
This wiring diagram is like that in
Figure 45
. It focuses on the recurrent projection from the corticospinal tract (CST) to the cerebellum via the pontine nucleus (PN), which forms a cerebrocerebellar communication loop (indicated by the circle with arrows). Abbreviations: SOT, spinoolivary tract; VL, ventrolateral thalamic nucleus. Other abbreviations and symbols are as shown in
Figure 45
. This system serves for voluntary movement control of a limb by the primary motor cortex as the controller.
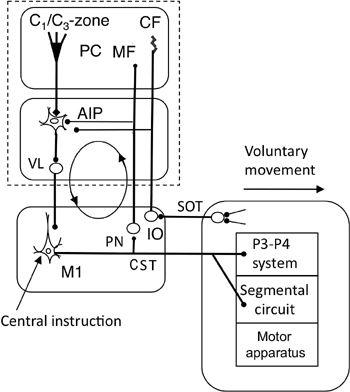
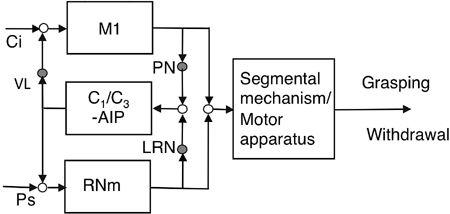
Figure 47. Hybrid control of a reflex and a voluntary movement.
A candidate example of the hybrid controller proposed in
Figure 9C
can be implied in the connections to both the magnocellular red nucleus (RNm) and the primary motor cortex (M1) from a microcomplex consisting of the C
1
/C
3
zones and the anterior interpositus nucleus (AIP). Other abbreviations are similar to those in
Figures 43
and
44
.
Because internal feedback predicts the sensory consequences of a movement, it can be used to cancel the actual effects of this movement, which otherwise would induce sensations that might disrupt the movement’s execution. A clear example of sensory cancellation has been observed in a fish “cerebellum-like” neuronal circuit. It has been shown to generate discharge that cancels the disruptive signals of lateral line organs caused by swimming (
Bell et al., 1997
). This mode of forward model control corresponds to the efference copy mechanism proposed earlier by
von Holst (
1954
). A unique example of sensory cancellation is that we perceive a self-generated tactile stimulus to be less ticklish than the same stimulus when it is applied externally by another means. In an fMRI experiment, Blakemore et al. (
1998
) revealed significantly less activity in bilateral secondary somatosensory cortices, the anterior lobe of the right cerebellum, and the anterior cingulate gyrus, for a tactile stimulus that was self- versus externally produced.
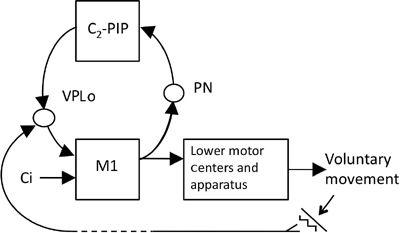
Sensory cancellation may have functional implications that are much broader than usually considered for tickling in humans and the sonar system of whales and bats, which may be located in their paraflocculus (recall
Chapter 2
). The reason is that the requirement of sensory cancellation is common to any voluntary movement that inevitably provokes disturbances created by the surroundings. How sensory cancellation occurs in neuronal circuits involving the cerebellum, however, is not yet known. On the basis of current information obtained in neuronal circuit analyses, however, I would like to suggest the possibility that a key role is played by the pathway sequentially connecting the primary motor cortex, pontine nucleus, C
2
zone, posterior interpositus nucleus, nucleus ventralis posterolateralis oralis (VPLo) of the thalamus, and then back to the primary motor cortex (
Figure 48
). A study using HRP tracing and single-cell recording showed that the VPLo relays sensory inputs from peripheral receptors to cells in the primary motor cortex (
Horne and Tracey, 1979
;
Tracey et al., 1980
). Sensory signals generated by tickling, for example, might be fed back via the VPLo to the primary motor cortex, whereas a microcomplex involving the C
2
zone and posterior interpositus nucleus would provide internal feedback. Signals of the two pathways would meet at the VPLo and cancel each other. This hypothesis is well worth testing because it suggests that the C
2
zone and posterior interpositus nucleus contribute not directly by modifying operation of the controller, but by participating in the sensory cancellation process that removes perturbations caused by voluntary movements. Caution is needed, however, because it is not yet known how the events in VPLo influence our perception.
Figure 48. A possible device for sensory cancellation.
The proposed device is a microcomplex consisting of the C
2
zone and the posterior interpositus nucleus (PIP). When the primary motor cortex (M1) responds to a central instruction (Ci) by commanding a voluntary movement, the command signals are also sent to the microcomplex via pontine nuclei (PN) and return from the microcomplex to M1 via the ventrolateral posterior oralis thalamic nucleus (VPLo), which receives somatic sensory signals from the periphery. A tentative hypothesis entertained in the text is that this circuit serves for sensory cancellation of the sensory perturbation induced by a voluntary movement.