The Flamingo’s Smile (9 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould

The medusa persons of a complex siphonophore include four basic types: swimming, floating, protection, and reproduction. The swimming organs, or nectophores, are minimally modified medusae—basically the upper swimming bells without the lower tentacles. Some siphonophores carry several orderly rows of nectophores; their rhythmic muscular contractions propel the creature, often in elaborate, looping trajectories. The passive floats, or pneumatophores, are filled with gas (of a composition near ordinary air) and maintain the siphonophore at the surface or at some intermediate depth. Their origin is a matter of controversy. Long interpreted as modified medusa persons, some biologists now regard pneumatophores as even more elaborately transformed polyps. The two most familiar siphonophores,
Velella
and
Physalia
, build large floats but contain no nectophores. They therefore move passively on winds and currents, often drifting into bays and beaches in vast accumulations.
The covering organs, or bracts, are the most curiously modified structures of all. They are usually flat, shaped like a prism or a leaf, and so different in form and function from a medusa person that we would scarcely suspect their origin if we could not follow their growth and budding.
The reproductive medusae, or gonophores, are budded off from polyp persons, the gonozooids discussed earlier. In a few species, gonophores are liberated to float in the ocean as independent objects. But they cannot feed, and die soon after releasing their sex cells. In most siphonophores, however, gonophores never separate from the parent colony and remain attached as a kind of sexual organ.
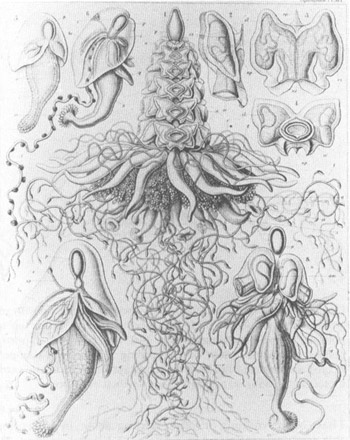
The middle figure shows a complete and complex siphonophore. The colony includes the following modified persons, from the top to the bottom: the single float, or pneumatophore (p); rows of swimming organs, or nectophores (n); fingerlike sensory projections, or palpons (q); clusters of reproductive parts (g); feeding siphons with trumpet-shaped mouths (s); and long, twisted strands of food-capturing filaments (t). Other figures are parts or early growth stages of the complex colony.
FROM E. HAECKEL’S
Challenger
MONOGRAPH,
1888.
REPRINTED FROM NATURAL HISTORY
.

One more complex siphonophore for good measure, and with yet another kind of person added (protective bracts). From top to bottom: a single pneumatophore; two vertical rows of nectophores; protective leaflike bracts; feeding siphons with trumpet-shaped mouths; and finally, long food-capturing filaments.
FROM E. HAECKEL
(1888).
REPRINTED FROM NATURAL HISTORY
.
The paradox of the Siphonophora expresses an issue that I have been avoiding, or rather skirting about, in presenting this taxonomy of persons or parts. I have described the various swimming, floating, protecting, feeding, capturing, and reproducing structures as persons—that is, as individual polyp or medusa organisms. Using evolutionary history as a criterion, this designation is almost surely correct and accepted by nearly all biologists. By history, siphonophores are colonies; they evolved from simpler aggregations of discrete organisms, each reasonably complete and able to perform a nearly full set of functions (as in modern coral colonies). But the colony has become so integrated, and the different persons so specialized in form and so subordinate to the whole, that the entire aggregation now functions as a single individual, or superorganism.
The persons of a siphonophore no longer maintain individuality in a functional sense. They are specialized for a single task and perform as organs of a larger entity. They do not look like organisms and could not survive as separate creatures. The entire colony works as a single being, and its parts (or persons) move in a coordinated manner. Although each nectophore (or swimming bell) maintains its own nervous system, a common nerve tract connects the entire set. Impulses along this pathway regulate the rows of nectophores in an integrated manner that permits the whole colony (or animal) to move with precision and grace. Touch the float of
Nanomia
at one end, and nectophores at the other extremity contract to remove the animal (or colony, if you will) from danger. Siphons pump their digested food along the common stem to the rest of the colony, but empty siphons also join in the general peristalsis, and food, as a result, reaches the entire colony (or organism) more effectively.
My studied parentheticals of the last paragraph underscore the fundamental paradox. Shall we call the entire siphonophore a colony or an organism—for it is a colony by evolutionary history but more an organism by current function. And what of the parts or persons? By history, they are modified individuals; by current function, they are organs of a larger entity. What is to be done?
This issue fueled the great siphonophore debate of nineteenth-century natural history. T.H. Huxley studied siphonophores during his long apprenticeship at sea on H.M.S.
Rattlesnake
(less celebrated than Darwin’s adventure on the
Beagle
, but another example of the same extended, exemplary, and largely extinct style of training in natural history). He interpreted siphonophores as conventional organisms, their parts as true organs and not modified persons. Huxley used siphonophores as his primary example in a famous essay on the nature of individuality in biology.
Louis Agassiz studied the Portuguese man-of-war on the shores of his adopted America (I have included his beautiful lithograph of
Physalia
with this essay) and decided that siphonophores are colonies, their integration a sign of divine handiwork.
Ernst Haeckel, artist and naturalist
extraordinaire
, described the siphonophores collected on that most celebrated of scientific expeditions in oceanography, the voyage of H.M.S.
Challenger
, 1873–1876. He published with his report a series of plates (including all other illustrations in this essay), unmatched ever since for beauty (though a bit short on accuracy, since Haeckel often added a touch of heightened symmetry for artistic effect). Haeckel also included several plates of siphonophores in his
Kunstformen der Natur (Art Forms in Nature)
of 1904—the great series of 100 lithographs, with plants and animals arranged in weirdly distorted form and swirling symmetry, in the best tradition of reigning
art nouveau
so well embodied in contemporary kiosks of the Paris Métro.
Haeckel’s theory of siphonophores would require an essay in itself to explain and explore, but he tried to mediate between Huxley and Agassiz by viewing these creatures in part as colonies (the poly-person theory in his words), in part as organisms (the poly-organ theory). Haeckel also used siphonophores, as Huxley had, to illustrate by dubious analogy his views on the proper organization of human societies. In his
Über Arbeitstheilung in Natur und Menschenleben (On the Division of Labor in Nature and Human Life)
, he compared the simple colonies of other cnidarians with the life styles of “primitive” humans and their limited division of labor for repetitive tasks performed by all: “The wild people of nature, who have remained on the lowest level right to our own day, lack both culture and division of labor—or they limit division of labor, as do most animals, to the different tasks of the two sexes.” He then compared complex colonies of siphonophores with the “advances” that division of labor permits in “higher” human societies—including modern warfare, where instruments of destruction “require hundreds of human hands, working in different ways and manners.”
Can we now suggest any resolution to this ancient debate, any possible mediation between two legitimate criteria that seem to yield opposite results—the criterion of history supporting the poly-person theory (siphonophores are colonies and their parts are persons) and the criterion of current function upholding the poly-organ theory (siphonophores are organisms and their parts are organs)? Can we tip the balance in favor of one view or the other by invoking the third major criterion of natural history—growth and form?
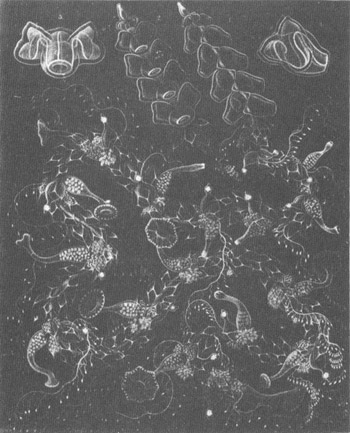
And finally, just for its aesthetic value, another Haeckel (1888) plate of complex siphonophores.
Growth and form provide us with an
embarras de richesses
by presenting evidence for and against both theories. As strong support for the poly-organ theory, siphonophores develop from a single fertilized egg cell. A siphonophore begins life as an unambiguous person—should we not regard any later development as an elaboration of this founding individual? Moreover, the adult siphonophore acts as a discrete object. Many species exhibit definite and complex symmetry governing all parts considered together. Some Portuguese men-of-war, for example, come in right- and left-handed versions.
We may, however, cite equally good arguments for the poly-person theory. Admittedly, each colony begins life as a single ovum, but it then develops a series of entities—full persons in this view—by budding from a common stem. This mode of growth is familiar in many aggregations conventionally regarded as colonies. A stand of bamboo may trace its origin to a single seed, yet we usually view each budded stem as an individual.
In addition, highly specialized structures sometimes bear vestigial parts that testify to their status as persons. In the poly-person theory, for example, nectophores are medusae that have lost all feeding and digestive parts, retaining only the jellyfish bell. But some nectophores grow rudimentary tentacles; in one species, the tentacles even retain eyespots. Protective bracts are the most modified and specialized of all siphonophore parts, but the bracts of two species retain a vestigial mouth—an indication that they arose as full medusa persons.
It looks like a tossup again. We might resolve our paradox if growth occurred in either of two ways—but nature doesn’t oblige. If all structures began growth as complete persons with a full set of parts, and then lost unneeded pieces as they specialized for swimming, protecting, or eating, then the poly-person theory would gain a big boost. If buds from the main stem began as complete persons and then disarticulated—the bell parts becoming nectophores and the tentacle parts siphons, for example—then the poly-organ theory would be affirmed. But most specialized parts simply grow as we find them. Nectophores differentiate as nectophores, bracts as bracts. We are immersed in an unresolvable conflict among equally legitimate criteria: discrete buds grow like a person with specialized parts like an organ. What, for example, shall we make of a gonophore, the degenerate reproductive medusa budded from a polyp? If it separates from the colony, we may choose to regard the gonophore as an organism. But it has no mouth and cannot feed; it must therefore die after releasing the sexual cells. Should we call such a limited breeding machine an individual? And if the gonophore remains attached to the colony, as it usually does, should we regard it as any more than a sexual organ?
When an inquiry becomes so convoluted, we must suspect that we are proceeding in the wrong way. We must return to go, change gears, and reformulate the problem, not pursue every new iota of information or nuance of argument in the old style, hoping all the time that our elusive solution simply awaits a crucial item, yet undiscovered.