The Rise and Fall of Modern Medicine (14 page)
Read The Rise and Fall of Modern Medicine Online
Authors: James Le Fanu

The idea of the pump was first conceived by John Gibbon in 1931, though almost a quarter of a century elapsed before he was in a position to perform his first open-heart operation. Gibbon's pump was not just any pump. Rather it made possible the most thrilling and eventful epoch in the whole history of surgery or, as one lyrical surgeon put it: âGibbon's idea and its elaboration take their place among the boldest and most successful feats of man's mind â the invention of the phonetic alphabet, the telephone or a Mozart symphony. Not a
deus ex machina
but a
machina a Deo
, a promethean fire; levelling from God his secrets to man's understanding; the pulse of the sacred heart; the breath of life.'
1
The pump transformed the operating theatre into a real theatre, a spectacle where the drama on the operating table was closely followed by an audience seated in rows behind glass panels and looking down at the action. And why was it so exciting? No surgeon before had been able to explore the interior of a living heart, while the technical problems of replacing valves and closing defects in the wall were not only the last but also the most sophisticated challenge a surgeon could hope to encounter.
The drama also lay in the literally life-or-death contest taking place on the operating table. The operations themselves were technically very difficult. The heart, though âopen', was, in the early days, still beating like a slippery eel while its small size in children (little more than a plum) made the job of repairing the defects particularly taxing. There was also the need to operate under the pressure of time, for even though the patient was âon bypass' the operation had to be done speedily to prevent long-term damage to the heart muscle. Come the end of the operation there was always the possibility that the heart would not recover its normal rhythm and the patient would die, not
discreetly in the wards several hours later, but there on the operating table while spectators seated in the gallery with their opera glasses looked on.
There was one further crucial element to this drama in the early days â the competition between the two main pioneers, Walton Lillehei and John Kirklin. It would have mattered less if they had been working in institutions a long distance apart but Walton Lillehei was based at the University of Minneapolis, just west of the Great Lakes, while ninety miles due south John Kirklin was Professor of Surgery at the internationally famous, massively well-endowed and prosperous Mayo Clinic in the town of Rochester. In the 1950s and 1960s every aspirant cardiac surgeon in the world flew to Minneapolis to watch Walton Lillehei at work, then hired a car or bought a train ticket and travelled south to see John Kirklin. It would have been difficult to avoid making comparisons but Lillehei and Kirklin were also utterly different in personality and style.
Before returning to the origins of open-heart surgery, a description by the world's first heart transplanter, Christiaan Barnard, of an event that took place while he was working as Lillehei's surgical assistant conveys some of the atmosphere of the daily life-and-(too often)-death struggle in the operating theatres in those early days:
Dr Lillehei was a great teacher . . . all of this was revealed one terrible day when I made an error in preparing a seven-year-old boy who had come to us for the repair of an imperfectly developed ventricular septum â or hole between the two lower chambers of the heart. He was a slender, dark haired boy from South America, and after we had him in position on the table I learnt his father was among those looking down at us from the glass dome above.
My job was to open the chest, expose the heart and then put tapes around two big veins bringing used venous blood to the heart. Once looped, the two veins would be hooked [via the plastic tubing] on to the heart/lung machine when Dr Lillehei arrived. Until he came, I was in charge, assisted by another doctor, Dr Derward Lepley.
There was trouble at the beginning, we opened the chest, exposed the heart and prepared to loop the two veins. The superior vena cava came into position easily. But in putting instruments around the inferior vena cava I found a bit of tissue in front of it. Turning to Dr Lepley, I gave the fatal command: âCut that, will you?'
He gave a cut with the scissors, but it was not quite enough. He cut again, and that was it. Blood spurted. We had cut into the heart.
âGive me an artery forceps â quick!'
I got it and tried to clamp the hole but only tore it further. The blood poured out now in a flood filling the cardiac cavity. The heart continued to beat, pumping its precious liquid, not into its own chambers but rather outside the heart itself. So it went on, like an animal drowning for want of help, until it was almost submerged and I could not see what I was doing.
âCall Dr Lillehei . . . now . . .'
As we sucked away at the blood the heart continued to pour out more of its own life, until the pressure started to fall. At this point the anaesthetist began to call out the awful figures [of the blood pressure].
âIt's below 80 . . . 70, now it's 65 . . .'
Frantically I reached my hand into the cavity filled with blood, trying to find the hole in the heart.
âWe are still going down . . . it's below 60 now, 53 . . . 42
and still descending . . . ' said the anaesthetist. Then he said, âI've got no reading. Pressure below 35.'
The heart had stopped. Blindly I reached in and began to massage it, hoping to start it again. But it did not respond and each successive squeeze of my hand only drew out more blood. I could not help but look up once, seeing the faces of those in the dome looking down at me including that of the father, his eyes wild with fear. Seeing me, he shook his head as though to say, âPlease say it is not true â say it is not my little boy, say it is not his heart that you have in your hand . . .'
Lillehei came and we connected the patient on the heart/lung machine. The cavity was drained and I saw where we had cut a hole into the left atrium. With the heart still not beating, but the boy held in life by the machine, Dr Lillehei began the operation opening the heart and repairing the leaking wall between the ventricles. After this he closed the hole we had cut into the upper chambers. Through it all I prayed the child would be all right â that when we ceased supporting him with the pump, the heart would again take over and sustain his life.
âAll right?' said Dr Lillehei finally. âLoosen it, let's see what we've got.'
The heart did not start despite massage and direct stimulants to the muscles. More stimulants were tried, but nothing could help. The boy was dead.
âClose the chest,' said Dr Lillehei, leaving the theatre and leaving me with Dr Lepley to stitch up the chest of the boy who only a few hours earlier had been alive and laughing and confident that he would soon be able to run and play with other boys. Now he lay, limp and dead, beneath my hands.
âI'm going,' said Lepley, leaving me to finish the job,
beneath the petrified gaze of the father in the dome above. I did not look at him. If I had I would not have been able to continue.
2
It is helpful in understanding the evolution of open-heart surgery to have a grasp of the workings of the heart, which consists of two sets of chambers â the right and left atria and ventricles lined up side by side. Venous blood from the upper and lower parts of the body drains into the large veins (the venae cavae) and is sucked first into the right atrium (hall) from where it is pumped into the right ventricle, from which it is propelled through the pulmonary valve into the pulmonary arteries, transporting it to the lungs, where it picks up oxygen and gets rid of carbon dioxide. The oxygenated blood then returns from the lungs to the left atrium, is squeezed through a valve into the muscular left ventricle and is then pumped out through the aorta and major vessels to be transported around the body.
With this scheme in mind, the development of cardiac surgery over the last fifty years can then be divided into four epochs. During the first â the 1930s and early 1940s â the heart was left undisturbed, but the large vessels rising from it, the pulmonary artery and aorta, were operated on to provide some relief of symptoms caused by defects within the heart. The second epoch started almost immediately after the Second World War when surgeons, having made an incision in the wall of the heart and with the heart still beating, âblindly' dilated narrowed valves with a knife or finger.
The crucial transition occurs in the early 1950s when, thanks to the pump, open-heart surgery becomes possible. There are many different defects in the heart that can only be repaired with open-heart surgery, and during the third epoch of cardiac surgery, from the mid-1950s to the early 1960s, surgeons started
to repair âholes in the heart' and replace diseased valves. The fourth and final epoch of cardiac surgery began in the late 1960s with the first heart transplant.
The most important transition was that from the âblind' and âclosed' operations to the âopen'. It will be illustrated by reference to a common type of heart defect in children, Fallot's Tetralogy, so named after the French physician Etienne Louis Fallot, who described the four (
tetra
) abnormalities of which two are of clinical importance (see page 105). Firstly, the pulmonary valve between the right ventricle and the pulmonary artery is narrowed, thus reducing the amount of blood that can be pumped from the right side of the heart into the lungs to pick up oxygen. Secondly, there is a hole in the wall between the ventricles through which the non-oxygenated blood from the right side of the heart is âshunted' into the left, thus bypassing the lungs. The consequence, as can be imagined, is that much of the blood being pumped from the left ventricle out into the main arteries has not passed through the lungs and so is not oxygenated. The child with Fallot's is thus blue (rather than pink) and breathless and grows poorly. These âblue babies' rarely lived beyond ten years of age. With the evolution of cardiac surgery, the results of treating blue babies becomes increasingly more dramatic, culminating in open-heart surgery, where a combination of dilating the narrow pulmonary valve and closing the hole in the heart restores the anatomy to normal.
The first phase of cardiac surgery started with a piece of lateral thinking by Dr Helen Taussig, a specialist in children's heart problems at Johns Hopkins Hospital in Baltimore. The main aorta, as it emerges from the left ventricle, passes in close proximity to the pulmonary arteries. It is thus quite straightforward, in theory, to link the aorta and pulmonary artery together so the non-oxygenated âblue' blood in the aorta passes
back through the lungs to pick up oxygen. Taussig had little difficulty in persuading the Professor of Surgery at Johns Hopkins, Alfred Blalock, about the soundness of her idea and the first operation was carried out on a fifteen-month-old baby boy in November 1944. He did not survive, but two further patients, aged eleven and six, were operated on the following year.
3
The Blalock/Taussig operation was an instant success and 500 operations were carried out over the following two years. Sir Russell, later Lord, Brock, the distinguished British cardiac surgeon, described the effects of the operation as âso outstanding that it altered the whole approach to cardiology'.
4
This then was the first phase â cardiac surgery without entering the heart. The second phase, âblind' operations within the heart, followed immediately. The simplest procedure for children with Fallot's is to dilate the narrow pulmonary valve between the right ventricle and the pulmonary artery, thus increasing the volume of blood that is pumped through the lungs. Here the surgeon is seeking to restore the natural haemodynamics of the heart, so dilating the pulmonary valve is a more elegant solution than the Blalock/Taussig operation. It does, however, require entering the heart by making an incision in the wall of the ventricle through which the surgeon can introduce a finger or knife, feel around for the narrowed valve and open it up. In 1948, this operation â a pulmonary valvotomy â was performed for the first time almost simultaneously by Russell Brock at Guy's and Holmes Sellors at the Middlesex Hospital, both in London.
5
,
6
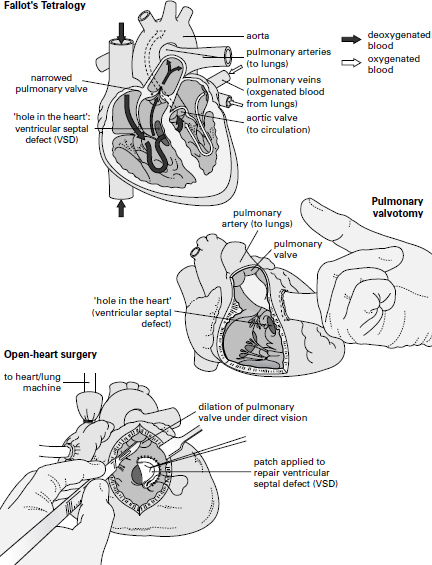
The two main anatomical defects of Fallot's Tetralogy are a narrowed pulmonary valve that limits the amount of blood pumped from the right side of the heart into the lungs, and a âhole in the heart' or ventricular septal defect, through which the deoxygenated blood from the right side is shunted through to the left. During a pulmonary valvotomy, the surgeon dilates the narrowed pulmonary valve through a small incision. With open-heart surgery, both the pulmonary valve can be dilated and the âhole in the heart' repaired, thus restoring to normal the internal anatomy of the heart.