Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (1095 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
7.7Mb size Format: txt, pdf, ePub
Serum osmolality measures the amount of chemicals dissolved in the blood. Chemicals that affect serum osmolality include sodium, chloride, bicarbonate, proteins, and glucose. A serum osmolality test is done to evaluate electrolyte and water balance. Serum osmolality is controlled partly by ADH or vasopressin. ADH is produced by the hypothalamus and is released by the pituitary gland into the blood.
Urine osmolality reflects the total number of osmotically active particles in the urine, without regard to the size or weight of the particles. Substances such as glucose, proteins, or dyes increase the urine specific gravity. Therefore, urine osmolality is a more accurate measurement of urine concentration than specific gravity, and urine osmolality can be compared with the serum osmolality to obtain an accurate picture of a patient’s fluid balance.

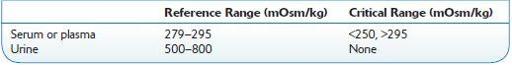
Normal range:
see Table 16.59.
TABLE 16–59. Normal Ranges for Osmolality
Use
Evaluate the balance between the water and the chemicals dissolved in blood.
Determine whether severe dehydration or overhydration is present.
Help determine if the hypothalamus is producing ADH normally.
Help determine the cause of seizures or coma. In severe cases, an imbalance between water and electrolytes in the body can cause seizures or coma.
Screen for the ingestion of certain poisons, such as isopropanol, methanol, or ethylene glycol.
Evaluate concentrating ability of the kidneys.
Evaluate electrolyte and water balance.
Used in workup for renal disease, SIADH, and diabetes insipidus.
Other books
Sputnik, mi amor by Haruki Murakami
Mr. Miracle (Harlequin Super Romance) by McSparren, Carolyn
Once Lost Lords (Royal Scales, Book 1) by Stephan Morse
Billionaire's Contract Engagement by Maya Banks
While England Sleeps by David Leavitt
Stealing Air by Trent Reedy, Trent Reedy
Sailing Deep by Noah Harris
Abyssinian Chronicles by Moses Isegawa
There Was an Old Woman by Ellery Queen
Frank: The True Story that Inspired the Movie by Jon Ronson