Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (626 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

Specimen validity is an important but often overlooked aspect of laboratory testing. Validity refers to the correct specimen identity (i.e., a urine sample is in fact human urine). The collectors of samples in physician offices and other sites have the responsibility of ensuring that adequate specimens are collected from patients. The validity of a specimen may be questioned if the sample is substituted or adulterated.
A
substituted
sample is a substance provided in place of the donor’s specimen. This may be drug-free urine (from another individual) or another liquid such as water.
An
adulterated
sample is a specimen to which substance(s) have been added to destroy the drug in the sample or interfere with the analytical tests utilized to detect drugs. Common additives include vinegar, bleach, liquid hand soap, lemon juice, and household cleaners. In the last several years, commercial products have become available to subvert drug tests. These products have been found to contain substances that include glutaraldehyde, sodium chloride, chromate, nitrite, surfactant, and peroxide/peroxidase.
In addition, specimens may be
diluted
by adding liquids to the urine at the time of collection to decrease the concentration of drug in the sample below the cutoff concentration used for the test. In vivo dilution involves the ingestion of diuretics and other substances to remove the drugs from the body or dilute the urine, for example, drinking an excessive quantity of water before a drug test.
Minimizing Specimen Validity Issues at the Collection Site
In many industries where urine is collected for drug testing for nonmedical purposes, for example, preemployment screening, specimens are collected under chain of custody and also several physical procedures are in place to minimize the likelihood of specimen substitution or adulteration. These procedures include witnessing the collection, not providing access to water in the bathroom, and coloring toilet bowl water. In addition, donors may not be permitted to wear loose clothing in which a substituted specimen could be hidden. Before the specimen is sealed in the collection container with tamper-resistant tape, the collector may record the temperature of the specimen (normal range for validity testing considered 90–100°F) as well as the urine color.
Urine Characteristics
Physical, chemical, or DNA tests may be used to assure the validity of a specimen. These tests are most frequently requested in connection with urine drug testing, especially for drugs of abuse such as cannabinoids (marijuana), heroin, and cocaine.
Creatinine, specific gravity, and pH are tests performed to assess whether a specimen is consistent with normal human urine. The U.S. Department of Health and Human Services Mandatory Guidelines specify acceptable ranges for these tests (for federally regulated drug testing specimens), and clinical laboratories and other providers have tended to adopt these values, some with slight modifications.
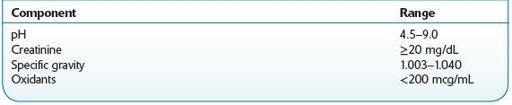
Creatinine is formed as a result of creatine metabolism in skeletal muscle, and the amount produced is relatively constant within an individual. This parameter is utilized clinically to assess renal function. A level ≥20 mg/dL in human urine is considered normal. Diluted and substituted (with water) specimens will have creatinine concentrations <20 mg/dL.
Specific gravity for liquids is the ratio of the density of a substance (urine) to the density of water at the same temperature. Hence, it is a measurement of the concentration of dissolved solids in the urine. The specific gravity of normal human urine ranges between 1.003 and 1.030. High values may be caused by disease (kidney disease, glucosuria, liver disease, dehydration, adrenal insufficiency, and proteinuria), whereas low values may be caused by diabetes insipidus (DI). Dilution and adulteration (e.g., with the organic solvents methanol and ethanol) results in a value <1.000.
The pH of normal human urine is typically between 5.0 and 8.0. The reference range is 4.5–9.0. This parameter can be affected by diet, medications, and disease. Acidic urine may be caused by acidosis (respiratory and metabolic), uremia, severe diarrhea, starvation, and diets with a large intake of acid-containing fruit. In contrast, an alkaline urine may be attributed to alkalosis, urinary tract infections, high-vegetable diets, and sodium bicarbonate. Individuals with dietary or disease causes of acidic or alkaline urine will be consistently in this range, rather than a random urine high or low due to adulteration or substitution. If lemon juice or vinegar is added to urine, the pH is lowered. A high urine pH results from the addition of bleach or soap.
Methodology
Tests should be conducted as soon as possible after urine collection. Creatinine may be determined by dipstick or automated clinical chemistry analyzer based on a chemical reaction that produces a color result. The specific gravity may be measured by refractometry in which the refractive index of the urine is determined. Alternatively, the dipstick method is based on ionic strength. A procedure available for automated clinical chemistry analyzers utilizes urine chloride ion concentration and spectrophotometry. pH is measured using a pH meter or colorimetrically manually or using an automated clinical chemistry analyzer.
Laboratories also offer tests for common adulterants. These include specific tests for nitrite and glutaraldehyde, typically using colorimetric assays, or generalized tests for oxidants. The latter detects compounds that exert their action by oxidation and include chromate and peroxidase. The method as performed on an automated clinical chemistry analyzer evaluates the reaction between a substrate and oxidant in the sample producing color that can be measured at a specific wavelength.
Specimen Requirements
A random urine specimen should be refrigerated after collection and sent to the laboratory as soon as possible. Urine samples suspected of bacterial contamination will produce invalid pH results. Sodium azide should not be used as a preservative, as this may cause interference with the Oxidant test. Table
14-4
presents reference ranges.
TABLE 14–4. Reference Ranges for Urine Specimens

THERAPEUTIC DRUG MONITORING
Purpose
Therapeutic drug monitoring (TDM) is the determination of drug levels in blood. The purpose of such measurement is to optimize the dose in order to achieve maximum clinical effect. TDM is typically performed on drugs with a low therapeutic index.
Indications
Signs of toxicity.
Therapeutic effect not obtained.
Suspected noncompliance.