Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (932 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
8.72Mb size Format: txt, pdf, ePub
A positive rapid fFN result may be observed for patients who have experienced cervical disruption caused by, but not limited to, events such as sexual intercourse, digital cervical examination, or vaginal probe ultrasound.
The rapid fFN result should always be used in conjunction with information available from the clinical evaluation of the patient and other diagnostic procedures such as cervical examination of a cervical microbiologic culture, assessment of uterine activity, and evaluation of other risk factors.
The assay has been optimized with specimens taken from the posterior fornix of the vagina or the ectocervical region of the external cervical os. Samples obtained from other locations should not be used.
Assay interference from the douches, white blood cells, red blood cells, bacteria, and bilirubin has not been ruled out.
Manipulation of the cervix may lead to false-positive results. Specimens should be obtained prior to digital examination or manipulation of the cervix.
Care must be taken not to contaminate the swab or cervicovaginal secretions with lubricants, soaps, or disinfectants (e.g., K-Y Jelly lubricant, Betadine disinfectant, Monistat cream). These substances may interfere with absorption of the specimen by the swab.
Patients with suspected or known placental abruption, placenta previa, or moderate or gross vaginal bleeding should not be tested for fFN.
FIRST-TRIMESTER SCREENING
See Prenatal Screening.
FLOW CYTOMETRY ANALYSIS IN THE CLINICAL EVALUATION OF HEMATOLOGIC DISEASES
*
Definition and use
Flow cytometry is a powerful tool for detailed analysis of complex populations in a short period of time. It integrates fluidics, optics, electronics, computer, software, and laser technologies in a single platform. It uses the principles of light scattering, light excitation, and emission of fluorochrome molecules to generate specific multiparameter data from particles and cells. Parameters measured by flow cytometry include intrinsic properties, such as forward scatter and side scatter, and extrinsic properties. Flow cytometric analysis has become a routine test in the diagnosis of hematologic diseases, including leukemias, lymphomas, plasma cell neoplasms, myeloproliferative neoplasms, myelodysplastic syndromes, and paroxysmal nocturnal hemolysis. Flow cytometric analysis can also be utilized in immunology, DNA analysis, and evaluation of genetic disorders. In addition, flow cytometric analysis can be used not only for diagnosis but also for disease prognosis and disease follow-up. A variety of clinical samples can be analyzed by flow cytometry, and they include peripheral blood, bone marrow aspirate, serous cavity fluid, cerebrospinal fluid, urine, fine needle aspirate, and solid tissue.
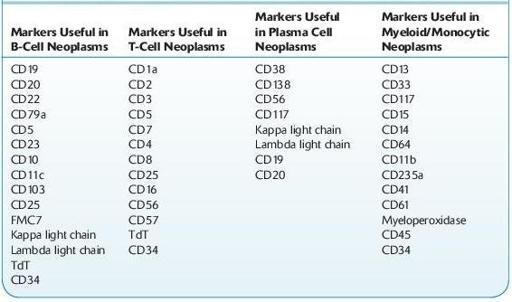
It is important to know about the commonly used immunologic markers and the design and utilization of flow cytometric panels in a variety of hematologic diseases, such as acute myeloid leukemia, acute lymphocytic leukemia, B-cell lymphoma, T-cell lymphoma, plasma cell neoplasm, paroxysmal nocturnal hemolysis, and myelodysplastic syndrome. Panel design is a critical step in flow cytometric analysis, and flow cytometric panels are designed to discern cell lineage, level of cell differentiation, and in many cases subclassification of the neoplasm being studied. Below are two tables with commonly used flow cytometry immunologic markers, which can provide practical guidelines to effective use of flow cytometric technology in the clinical laboratory. For specific flow cytometric findings in particular diseases, please refer to Chapter of “Hematologic Diseases.”
Useful Flow Cytometric Immunologic Markers in Hematologic Neoplasms
Other books
The Daddy Decision by Donna Sterling
Christmas in Eternity Springs by Emily March
Nipped in the Bud by Susan Sleeman
Blessings of the Heart by Valerie Hansen
To Love a Way of Life by Natalie Hart
The Lake (The Lake Trilogy, Book 1) by Grant, AnnaLisa
Louisiana Laydown by Jon Sharpe
Supernova by Jessica Marting
Nowhere but Up by Pattie Mallette, with A. J. Gregory
Brothers Black: Wyatt the Heartbreaker by Blue Saffire