Alien Universe (29 page)
Authors: Don Lincoln

Silicon-Based Life?
In science fiction, there is soft SciFi and hard SciFi. In hard SciFi, the writer tries to advance the plot line constrained by the best-known science of the time, while in soft SciFi, more liberties are taken with the science. In the case of stories about alien life, a common alternative to our familiar type of life is one based on the silicon atom. The arguments presented earlier about the advantages of carbon (specifically the four bonds available and the rich
chemical complexity that comes with it) are rather compelling, suggesting the four available bonds are a necessary condition of complex life. In fact, chemists have cataloged more molecules involving carbon than all the known molecules that exclude carbon. Think about that. If you took all elements except carbon and made every known compound, you’d have fewer compounds than the ones that have been found and contain carbon.
Given the benefits of four bonds, it is therefore natural that a hard SciFi writer who wants to break away from carbon-based life would then invoke silicon as the next candidate base element around which to build a fictional ecosystem. There’s only one problem: it isn’t as simple as that.
We’ve already noted the simple objection that while we breathe out carbon dioxide as a gaseous waste product, silicon dioxide is solid and we are more familiar with it as sand. This particular fact was noted early on in the 1934 short story
A Martian Odyssey
, by Stanley G. Weinbaum, in which he described a Martian silicon-based creature that excretes bricks every ten minutes. These bricks were the waste products of respiration.
However, the problems with silicon are much deeper and fundamental than this. Far more damaging are silicon’s issues with its stability in its interactions with other atoms and the rate at which silicon chemically interacts.
A very important feature of how carbon bonds with other elements is that the bond strength between two carbon atoms (C–C) is quite similar to that of a carbon-hydrogen bond (C–H), as well as carbon-oxygen (C–O) and carbon-nitrogen (C–N). Because of this, it is energetically fairly easy for a reaction to swap out one atom and connect another. From an energy point of view, which of these elements participate in the bond doesn’t matter much and so these swaps occur pretty freely.
In contrast, silicon doesn’t have this property. It turns out that silicon-oxygen (Si–O) bonding is much stronger than with hydrogen (Si–H), nitrogen (Si–N), or even other silicon atoms (Si–Si). Consequently, silicon binds easily to oxygen (making silicon dioxide), and it is very hard to break apart that bond and slip in another atom.
What we’ve mentioned here is just a characteristic of single interatomic bonds. When we turn our attention to multiple bonds, carbon is again quite superior. It turns out that a double carbon bond takes about twice as much energy as a single bond, while a triple bond uses about three times as much energy. It didn’t have to be that way. The details of multiple bonds are different from single bonds, and carbon just got lucky.
Silicon, in comparison, has a much harder time making double and triple bonds. This has to do with the size and shapes of the atoms. The pictures of
figure 6.5
give an overly simplified impression of the shape of atoms. Silicon and carbon really look like spheres with bumps protruding out of them, with the bumps participating in the bonds. Because the silicon sphere is bigger than the carbon one, and the silicon bumps aren’t much bigger than the carbon ones, the bumps are farther away between two adjacent silicon atoms. This makes it harder to get the bumps closer to other atoms to share electrons, which makes a second bond much weaker than the first one. Consequently, the strength of double bonds between adjacent silicon atoms aren’t much different from single silicon bonds. This makes complex chemistry using silicon that much harder. This point is illustrated in
figure 6.12
.
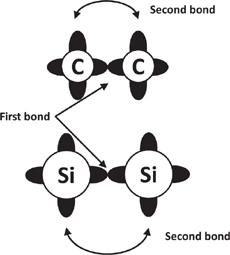
FIGURE 6.12
.
Because of their size and shape, silicon atoms have a hard time making stable double and triple bonds. The strength of the second silicon bond is much weaker than the first silicon bond. This is in contrast to carbon, in which the second bond is comparable to the strength of the first bond. The black areas represent electrons available for bonding. In silicon, the electrons participating in the second, third, and fourth bonds are separated by a greater distance and consequently bond more weakly.
Finally, the ease at which reactions can occur is much greater with silicon atoms. Consider a gas stove, inadvertently left on, so carbon-containing natural gas fills the house. The gas can fill the house, but it won’t explode without a spark to set events in motion. However, a similar “silicon natural gas” would spontaneously react without the spark. This speed of reaction reduces the time necessary to form complex molecules.
So does this mean that silicon-based life is impossible? Could the rock people of planet X be having a discussion about the benefits of silicon-based life? Well, sure. It’s not like the factors mentioned in this chapter are definitive, nor should you think that we’ve exhaustively explored all options. But these factors are certainly strong reasons to not think of silicon-based life as equally likely as other worlds covered with carbon-based life. Even Carl Sagan was reported to have stated that while he was only a weak water chauvinist, he was a huge carbon chauvinist.
So scientists must consider the possibility of alien life based on atoms other than carbon, but it isn’t considered to be highly likely. However, when we talk in this way about silicon life, we need to remember that we’ve been talking about life that evolved directly from non-living substances. There is another form of silicon life that we should keep in mind.
Second-Generation Silicon
“Resistance is futile. You will be assimilated.” This is one of the trademark phrases of one of the nemeses of humanity in
Star Trek: The Next Generation
. The Borg are cyborgs, which are a mixture of organic (i.e., squishy stuff like us) and cybernetic implants, which obviously include metals and silicon. In Fred Saberhagen’s
Beserker
series, self-replicating robotic creatures roamed the cosmos, intent on destroying life. A computer called HAL in
2001: A Space Odyssey
became self-aware and turned on his crew. The eponymous Terminator is a self-aware robot tasked with the termination of humanity. The Cylons of
Battlestar Galactica
are at war with humans. The Daleks of
Dr. Who
wander around saying “exterminate.” The silicon-based creatures of science fiction are often bad guys.
One can find many examples of cybernetic enemies of humanity in the science fiction literature. The story line is often similar to that of Frankenstein, when an artificial form of life gets out of control and turns on its creator. However organisms of this form must be considered life in the sense of how we mean Aliens. These cybernetic creatures (whether enemies or friends) would not have directly evolved from inanimate matter, but we should keep them in mind as we consider what sort of Aliens we might one day encounter. Indeed, when one considers a second-generation form of life—meaning one that is carefully designed by a first form of intelligent life (where by first form, I mean a type that has evolved from scratch)—many of the considerations listed here are less important. Metals, silicon, other elements could easily be essential parts of created life. Even second-generation carbon-based life could have a more complex and efficient biochemistry.
But, really, the idea of second-generation life is perhaps not the first concern for scientists looking for Aliens in the universe. However, if Alien spaceships ever appear over the cities of Earth, it’s probably best to hope that they aren’t in the form of big cubes. You know … just in case …
Wrap Up
While I’ve tried in this chapter to describe the most important considerations in the creation of life, you should by no means think that what I’ve said here is airtight. Some of the things are pretty inarguable, for instance, it seems exceedingly unlikely that helium will play a huge role in the biochemistry of Aliens. Helium just doesn’t participate in atomic bonding. Further, there is a clear advantage to the use of carbon as a base element. Being able to create many bonds leads to a complex chemistry and a correspondingly diverse biology. It is also true that without adequate energy (and an exploitable energy difference), life cannot exist.
However, beyond that, it is hard to say anything definitive. Once one gets past the minimal chemical and physical considerations of life, evolution is a powerful optimizing tool. Earth-based biochemical cycles are extremely complex, and it is literally unbelievable that extraterrestrial biochemistry will not be both as complicated and different than the paths observed on Earth.
Still, we know enough about chemistry to know that some possible metabolic pathways cannot yield the same amount of energy as others. This does set some limits on the Aliens we might encounter. However, when we take into account that life might exist on planets with very different temperature or pressure than we find on Earth, the limitations are not quite as absolute as it may seem.
What I hope I’ve done is to have given you a sense that not all of the ideas you might encounter in science fiction are possible, for instance a sentient gas cloud is pretty hard to imagine. Still, the realm of the possible is still rather broad. Astrobiologists definitely have their work cut out for them.
SEVEN
NEIGHBORS
Sometimes I think the surest sign that intelligent life exists elsewhere in the universe is that none of it has tried to contact us.
Bill Watterson,
Calvin and Hobbes
Thus far, we have discussed mankind’s vision of Aliens without much attention to the contribution of modern observational astronomy. The first musings about the surface of Mars by Percival Lowell and his contemporaries were informed by the best science of the time, although we recall that many scientists dismissed his beliefs as ridiculous. However, you should recall that during that era, there were ongoing, multidecade arguments over the existence of canals on the face of Mars. These canals were reported to be thousands of miles long and to irrigate swaths of the planet’s surface tens or even hundreds of miles wide. This tells us something about the technology available to those early scientists. By today’s standards, it was crude. If cautious and sober scientists could imagine observing an extensive canal system on our nearest planetary neighbor, it was quite unrealistic for scientists of the era to have a more detailed and accurate picture of possible life on Mars.
This is not to say that the scientists of the early twentieth century didn’t exploit all the instruments at their disposal. As we mentioned in
chapter 1
, those same scientists did have access to spectroscopy and so they were able to make crude measurements of the composition of some planetary atmospheres and determine the presence of substances vital to earthly life, like oxygen and water. For instance, most scientists of that era were aware that Mars was dry (hence the reason that the canal mythology resonated so well) and had very little oxygen. However, these early insights were but a first step along the path to modern planetology.
Continuing our review of what we have covered so far, in
chapter 2
we looked at the impact of reports of extraterrestrials, mostly by observers who were not well-informed of the science of the day. Their stories, totally irrespective of whether they were objectively true reports or not, incorporated culturally familiar religious themes (Adamski) and nightmarish ones (Betty and Barney Hill). The science of the middle of the twentieth century mattered less to the public’s vision of Aliens than did these UFO reports.
The science fiction discussed in
chapters 3
and
4
is, by definition, speculative. Often science fiction writers have a respectable knowledge of contemporary scientific thought, but their goal is to tell a tale; often one that says more about humanity than it does about science or real Alien behavior. We should not trust these stories to be bound by the rigid strictures of the best scientific knowledge.
Even when we turned to more scientific thinking in
chapters 5
and
6
, this discussion was more about understanding the range of the possible, informed by what we have found here on Earth and later by the limitations imposed by the physical laws of the universe. While these are valuable lessons, what could be and what actually is are quite different things. Humans could have evolved to be 9 feet tall or could have descended from a nonprimate lineage. Neither turned out to be what happened.
Accordingly, in order to understand what real, true Aliens are, the only way we’ll ever know the definitive answer is to either go and meet them and shake their hand or tentacle or whatever greeting is appropriate or talk to them somehow. Since we have no hard evidence that Aliens exist (the reports of
chapter 2
notwithstanding), what do we actually know? What has science learned about the existence of extraterrestrial life (and, more importantly, Aliens)? What do we know about the probability of encountering life if we ever explore the galaxy?