Eight Little Piggies (11 page)
Read Eight Little Piggies Online
Authors: Stephen Jay Gould

If each organ had only one function (performed with exquisite perfection), then evolution would generate no elaborate structures, and bacteria would rule the world. Complex creatures exist by virtue of slop, multiple use, and redundancy. The hyomandibular, once a gill support, then evolved to brace jaw and braincase. But this bone happens to lie right next to the otic capsule of the inner ear—and bone, for reasons incidental to its evolution, can transmit sound with reasonable efficiency. Thus, while functioning primarily as a brace, the hyomandibular also acquired other uses. Skates and rays take in water through a round opening, called the spiracle, located in front of the other gill slits. The hyomandibular then helps to pump this water into the mouth cavity, and thence out and over the gill slits. Closer to our phyletic home, the hyomandibular may help to ventilate the lungs of modern lungfishes.
I have wanted to write about the origin of middle-ear bones ever since I began this series, for we have no finer story in vertebrate evolution. But I like to wait for a handle in new information, and one recently came my way (see J. A. Clack, in bibliography, on finding the oldest stapes). The first known tetrapods (four-legged terrestrial vertebrates) hail from eastern Greenland in rocks 360 million years old (see Essay 4). They have been known for some time under the names
Ichthyostega
and
Acanthostega
, but their stapes had not been well resolved before. Clack found six stapes of
Acanthostega
, four preserved in their life positions.
Clack suggests not only a dual but a triple function for the stapes of these first land vertebrates. The bone is stout and dense, not slender and delicate as in stapes adapted largely for hearing. This original stapes must still have functioned in its earlier role as a brace (other early tetrapods, including mammalian ancestors, also had stout stapes). Clack also advocates a supplementary role in respiration. Finally, she makes a key observation based on the stapes’s location: “The stapes is likely to have had some auditory function because of the close association between the footplate [a part of the stapes] and the otic [ear] capsule.”
Such a multifarious bone nearly bursts with evolutionary potential. The stapes may have braced for a hundred million years, but it also worked for respiration and hearing if only in an incipient or supplementary way. When the cranium later lost its earlier mobility, and the braincase became firmly sutured to the skull—as occurred independently in several lineages of terrestrial vertebrates—the stapes, no longer needed for support, used its leverage and amplified a previously minor role in hearing to a full-time occupation.
2.
The origin of mammalian middle-ear bones
. The odyssey of the stapes (stirrup) is extraordinary enough, a tale worthy of Scylla, Charybdis, and all the wiles of Circe—from gill support to a brace between jaws and braincase to a hearing bone for airborne sound. Yet the other two bones of the mammalian middle ear, named long ago by an age that knew the blacksmith’s forge, have an even more curious history. The hammer and anvil (malleus and incus), as elements of the gill arch in front of the hyomandibular, became parts of the jaw in early vertebrates. In fact, they took up the central role of connecting and articulating the upper and lower jaws—as they still do in modern amphibians, reptiles, and birds. The quadrate bone of the reptilian upper jaw became the incus of mammals, while the articular bone of the lower jaw became the malleus. The transition, so improbable in bold words, is beautifully documented in the fossil record and in the embryology of all modern mammals.
The homology of reptilian jawbones to mammalian ear bones was discovered by German anatomists and embryologists well before the advent of evolutionary theory. In 1837, C. B. Reichert made the key observations and expressed the surprise that this tale has elicited ever since. With these words, Reichert introduced his section on the
Entwicklungsgeschichte der Gehörknöchelchen
(developmental history of the little hearing bones). (German looks so god-awful for its massive words. But these tongue twisters are usually made of little words compounded, and the system becomes beautifully transparent, even charming, once you break the big items into their elements. The Germans have preferred to construct technical terms as compounds of their ordinary words, rather than from fancy and foreign Latin or Greek. A rhinoceros is a
Nashorn
, or “nose horn” as rhinoceros actually says in Greek; a square is a
Viereck
, or four-corner. Our technical literature refers to the hammer, anvil, and stirrup as “auditory ossicles” don’t you prefer the German
Gehörknöchelchen
, or little hearing bones?) In any case, Reichert wrote: “Seldom have we met a case, in any part of animal organization, in which the original form of an early [embryological] condition undergoes such extensive change as in the ear bones of mammals. We would scarcely believe it…. Nevertheless, it happens in fact.”
Reichert recognized all key outlines of the story: that all the ear bones derive from the first two sets of gill-arch bones, the hammer and anvil from the first arch (forming the jaw of vertebrates), and the stirrup from the second arch (forming the hyomandibular of fishes). He noted that the lower jaw first forms with a precursor called Meckel’s cartilage (in honor of another great German anatomist of the generation just before, J. F. Meckel). The mandible or jawbone then ossifies on the side of Meckel’s cartilage. Meanwhile, the posterior end of Meckel’s cartilage, forming the back end of the jaw in the early pig embryo, ossifies and then detaches to become the malleus of the middle ear. One could hardly ask for more direct evidence, and Reichert’s observations have been affirmed thousands of times since.
Two illustrations from Reichert’s classic article of 1837, containing his discovery of the homologies of mammalian middle-ear bones. See text for explanation.
Courtesy of Department of Library Services, American Museum of Natural History
.
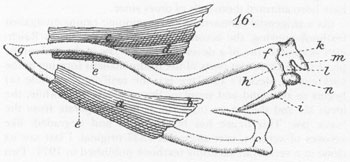
(As a tangential comment in my continuing campaign against textbook copying, the accompanying illustration shows Reichert’s original figure of a developing lower jaw in the embryonic pig;
h
and
i
represent parts of the future malleus forming at the back end of Meckel’s cartilage (
g
); the ossifying mandible (
a
) begins to surround and supplant the cartilage. Meanwhile, the incus (
k
) and the stapes (
n
) form as bones separate from the lower jaw. This figure has been copied and degraded, like xeroxes of xeroxes, ever since this 1837 original. I last saw its clone in a vertebrate anatomy textbook published in 1971. Two bits says that the author of this text [who undoubtedly copied the figure from a book just slightly older than his] would be shocked to learn that his picture dates from 1837. This time, everyone lucks out because Reichert was a great anatomist and his figure is basically correct; but think of the capacity for compounded error inherent in this procedure of mindless copying. I also include, to give an interesting [if gory] flavor of common styles of illustration during the early nineteenth century, one of Reichert’s graphic preparations of a pig embryo, dissecting pins and all.)
Thus, every mammal records in its own embryonic growth the developmental pathway that led from jawbones to ear bones in its evolutionary history. In placental mammals, the process is complete at birth, but marsupials play history postnatally, for a tiny kangaroo or opossum enters its mother’s pouch with future ear bones still attached to, and articulating, the jaws. The bones detach, move into the ear, and the new jaw joint forms—all during early life within the maternal pouch.
Paleontological and functional evidence join the embryological data to construct a firm tripod of support, giving this narrative pride of place among all transitions in the evolution of vertebrates by combining strength of documentation with fascination of content. One theme stands as the coordinating feature of this narrative (and of my entire essay): redundancy and multiple use as the handmaidens of creativity.
We might employ this theme to make an abstract prediction about the character of intermediary forms in the fossil record. Contrary to creationist claims that such a transition cannot occur in principle because hapless in-betweens would be left without a jaw hinge, the principle of redundancy suggests an obvious solution. Modern mammals hinge their jaws between squamosal (upper jaw) and dentary (lower jaw) bones; other vertebrates between quadrate (upper jaw) and articular (lower jaw) bones destined to become the incus and malleus of the mammalian ear. Suppose that mammalian ancestors developed a dentary-squamosal joint while the old quadrate-articular connection still functioned—producing an intermediary form with a double jaw joint. The old quadrate-articular joint could then be abandoned, as its elements moved to the ear, while the jaw continued to function perfectly well with the new linkage already in place.
Our woefully inadequate fossil record is not brimming with intermediary forms, for reasons often discussed in these essays. But the origin of mammals represents a happy case of abundant evidence. The abstract predictions of the last paragraph (actually advanced by paleontologists before the discoveries, so I am not just making a rhetorical point here) have been brilliantly verified in abundant fossil bone. The cynodont therapsids, our ancestral group among the so-called mammallike reptiles, show numerous trends to reduction and loosening of both quadrate and articular bones in the old reptilian jaw joint. Meanwhile, the dentary of the lower jaw enlarges and extends back to contact the upper jaw. (In mammals, the dentary forms the entire lower jaw; reptilian jaws contain several postdentary elements, all reduced and then suppressed or dispersed in mammals.) Many cynodonts develop a second articulation between the squamosal and a postdentary element of the lower jaw called the surangular. (This joint is not the later mammalian dentary-squamosal link, but its formation illustrates a multiple evolution of the intermediacy proclaimed impossible by creationists.) Finally, two or three genera of advanced cynodonts develop a second articulation of truly mammalian character between the dentary and squamosal. One such genus (although the evidence has been disputed) bears the lovely and distinctive name
Diarthrognathus
, or two-jointed jaw.
Moreover, the earliest true mammals do not yet have a fully independent malleus and incus. These bones remain affixed to the jaws and continue to participate in articulation, in both
Morganucodon
and
Kuehneotherium
, the two best known early mammals. “In this sense,” wrote Edgar F. Allin in 1975, “the earliest mammals did not yet possess a ‘mammalian middle ear.’” By Upper Jurassic times, still well within the early days of mammalian life in a world dominated by dinosaurs, these bones had entered the ear, and an exclusively dentary-squamosal joint had formed.
Embryology and paleontology provide adequate documentation of the “how,” but we would also like more insight into the “why.” In particular, why should such a transition occur—especially since the single-boned stapedial ear seems to function quite adequately (and, at least in some birds, every bit as well as the three-boned mammalian ear)? We are nowhere near the full answer to this complex question, but one hint conveys special interest and also illustrates the principle of redundancy one more time.
Pelycosaurs, those sail-backed creatures included in every set of plastic dinosaurs and every box of chocosaurus cookies, are not dinosaurs at all, but our distant ancestors—forebears of the therapsid reptiles that eventually evolved into mammals. The stapes of pelycosaurs lies in close contact with the quadrate bone of the upper jaw (forerunner of the incus that now articulates with the stapes in the mammalian middle ear). This linkage continues and sometimes intensifies in descendant therapsids—the more immediate ancestors of mammals. This anatomical connection strongly suggests that the quadrate of mammalian ancestors, while functioning primarily in jaw articulation, already played a subsidiary role in the transmission of sound. Allin argues: “From the nature of its junction with the stapes, the cynodont quadrate obviously took part in sound conduction.”