Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (909 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
13.88Mb size Format: txt, pdf, ePub
Other names: estrogen receptor assay (ERA), progesterone receptor assay (PGRA), progesterone receptor protein (PRP), estrogen receptor protein (ERP).

Normal range
(ERP, PRP):
Negative: <5% of nuclei staining
Borderline: 5–19% of nuclei staining
Positive: 20% of nuclei staining
Use
To identify patients with breast cancers likely to respond to either additive or ablative hormone therapies
Interpretation (Table 16.30)
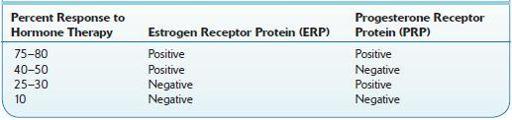
TABLE 16–30. Percentage of Patients Who Respond to Hormone Therapy Based on the Test Results of Estrogen and Progesterone Receptor Assay
Limitations
Assay is performed on paraffin-embedded, formalin-fixed tissue.
Receptor status is influenced by age.
The definition of positive and negative may vary from laboratory to laboratory due to tissue and antibody treatment and antibody specificity.
Suggested Reading
Ogawa Y, Moriya T, Kato Y, et al. Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: Analysis for a cut-off point as the predictor for endocrine therapy.
Breast Cancer.
2004;11(3):267–275.
ESTROGENS (TOTAL), SERUM
Definition
Other books
Man-Kzin Wars XIII-ARC by Larry Niven
A Faraway Island by Annika Thor
Undeniably Yours by Heather Webber
Unstuck by Liliana Camarena
Andrea Pickens - [Lessons in Love 01] by The Defiant Governess
Blue Knickers, A Spanking Short by Rodney C. Johnson
Hold: Hold & Hide Book 1 by Grey, Marilyn
Linda Castle by Temple's Prize
Cheeseburger Subversive by Richard Scarsbrook
The Last Trade by James Conway