Why Is Milk White? (6 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Ammonium hydroxide is a strong alkali that reacts with fats and oils in much the same way lye does, forming water-soluble soap. It is used in glass cleaning products to remove grease and oils from windows.
Alcohols dissolve grease and fats, and one alcohol, isopropanol, is used to remove grease from skin and to disinfect the skin (it is sometimes called rubbing alcohol). It is added to window cleaners to help remove oils and grease.
The main detergents in most shampoos are sodium lauryl sulfate and sodium lauryl ether sulfate (sometimes called sodium laureth sulfate). These are white solids or powders in their pure form, and when dissolved in water they form a thick liquid that is very slightly off-white, like lemonade.
Shampoo manufacturers generally like prettier colors than that. They may add natural colors like tea extracts and plant dyes such as henna, beta carotene, and annatto, but these may require additional preservatives to keep them from spoiling during storage.
The Federal Food, Drug, and Cosmetic Act regulates food and cosmetic colorings, among other things. Some colors do not require certification by the FD&C because they are “Generally Recognized As Safe,” or GRAS. These include such things as caramel color, beet juice, cochineal extract, saffron, mica, and beta carotene.
Unlike the colors that do not need to be certified, which contain a mixture of different compounds, each FD&C certified color is a pure compound. There are seven lists of colors. The first list is colors that are allowed in foods. The second list can be used in drugs and cosmetics. The third list is for only externally applied drugs and cosmetics. The other lists either exempt colors or add further restrictions.
The first list contains the FD&C colors. You may have seen these in the ingredients list on foods you have eaten.
- FD&C Blue #1
- FD&C Blue #2
- FD&C Green #3
- FD&C Red #3
- FD&C Red #40
- FD&C Yellow #5
- FD&C Yellow #6
The colors in the second list are not to be used in foods, and so the F is not included in the name. Some examples are:
- D&C Green #5
- D&C Orange #5
- D&C Red #6
- D&C Red #7
- D&C Red #21
- D&C Red #22
- D&C Red #28
- D&C Red #30
- D&C Red #33
- D&C Red #36
- D&C Yellow #10
Carmine (also known as cochineal extract) is one color that may surprise many people. It is made from the body and legs of a female scale insect that lives on cactus plants. Almost onequarter of the dry weight of these insects is carminic acid, which the insects produce to prevent other insects from eating them. This is mixed with aluminum or calcium salts to make the red dye called carmine. You have probably eaten something colored with this dye, made from little tiny bugs.
It isn't. It often is colored using the same colors that are used for shampoo. But before getting added colors, conditioner is white, and opaque. It is white and opaque for the same reason milk is white and opaque. Like milk, it has tiny droplets of oils and fats surrounded by water. These droplets scatter the light that hits it, just like the droplets of water in a cloud do. We see the result as opaque and white.
Hair conditioner has several ingredients in it that each do their own task to condition the hair. Hair has millions of tiny scales on each strand, and if these scales stand up away from the hair, it makes the hair tangle more easily and appear dull.
Acids, like citric acid, are added to conditioner because the acid makes the scales lie down against the hair.
Conditioner also has some detergents like shampoo does. But the detergents in conditioner are designed to stay in the hair. The water-loving end of the detergent sticks to the protein the hair is made of. The other end of the detergent is a long chain that sticks to oils and fats. This allows the detergent to hold on to the hair and
the oil at the same time, so the oil coats the hair. This makes the hair shiny and helps prevent tangles.
One of the detergents used is
panthenol,
a precursor chemical to vitamin B
5
(pantothenic acid). It holds on to the hair protein and also holds on to water. Besides coating the hair and making it shiny, it also lubricates it to prevent tangles. It is sometimes called a
provitamin,
but it has no nutritive value when added to hair, which is just protein and has no living cells.
Other anti-tangle ingredients are silicone-based lubricants, such as dimethicone, and fatty alcohols (synthetic detergents made from oils). When you see stearyl alcohol or cetyl alcohol, those are fatty alcohols. Cetearyl alcohol is a mixture of the two. They help make the conditioner opaque, in addition to their lubricating and stabilizing functions.
Shampoo without any perfumes would smell like the detergents it is made of. This is not a particularly pleasant smell. Even “unscented” products usually include a
masking scent,
which is a perfume designed to hide the odor of the other ingredients without having a lingering effect in the hair.
Coming up with a good perfume scent for a shampoo involves overcoming several obstaclesâcost, stability, safety, colorâand optimizing for the desired characteristicsâa unique scent, how well the hair holds the scent, how the shampoo smells in the bottle, or how long the scent lasts.
With over 3,000 perfume elements to choose from, the perfumer will choose the much smaller set of scents desired for the shampoo. For example, it turns out that more people like fruitysmelling shampoos than other scents. The chemist will then select from that list the scents that work well with the other ingredients in the shampoo. Some scents will be destroyed or changed by the detergent and can't be used. Other scents won't dissolve well in the shampoo, or they won't release from the shampoo well enough to be detected. Some of them will stick to the hair well, and others won't.
PAPER CHROMATOGRAPHY
Materials
Food coloring (yellow, red, and green)
Paper towel
Jar
1
teaspoon salt
2 cups water
Wire coat hanger
Binder clip
¼
cup rubbing alcohol (optional)
¼
cup nail polish remover or acetone (optional)
Chemists have many ways to separate mixtures of compounds into their individual components. But few are as colorful and simple as paper chromatography.
You may have seen or even done paper chromatography, but this project goes into a little more detail than is usually seen in popular books and websites.
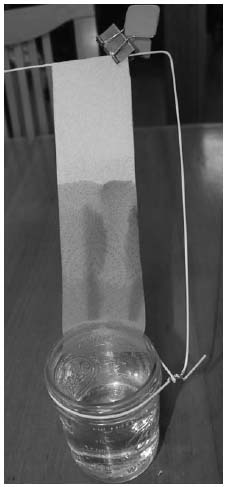
The photo on the next page shows four spots of food coloring on a strip of paper towel: yellow, red, and green, as well as a spot made of all three colors combined. The end of the paper is resting in a jar of 1 percent saltwater solution (1 teaspoon of salt in 2 cups of water). As the salt water wicks up into the towel, it carries some of the dye molecules faster than it does others. Pure water could be used, but the salt water helps make some compounds move faster up the paper than others.

The entire setup is shown on the right. A bit of wire from a coat hanger and a binder clip support the paper towel, and you can see the colors have separated as they climbed up the paper.
On the next page, the different areas are marked with labels. The box of food coloring that was used says it contains the colors FD&C Yellow #5, FD&C Blue #1, FD&C Red #40, and FD&C Red #3. Notice that there is no green color listed, and there are two different colors of red.
The two reds show up as separate areas of color. The bottom one is a slightly
magenta red, and the upper one is a slightly orange red. Combined, they make the color that the dye manufacturer wanted, a rich solid red color.
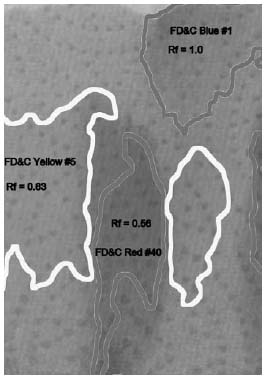
The green food coloring has also separated into two patches of color. The blue color climbed the towel quickly, staying with the very top of the water that wicked up into the towel. Below it is a patch of yellow, still stained faintly with some of the blue, so it appears a little greener than the pure yellow in the first column.
In the last column, three of the four colors have separated. The yellow and one of the reds seem to climb the paper at the same rate, and stay mixed. But the blue raced to the top, and the magenta red lagged behind, barely moving.
The speed of the molecules relative to the salt water is called the
retardation factor,
or Rf. It is simply the distance the color has moved up the towel divided by the distance the water has moved. We use an estimate of where the center of the color spot is for the calculation.
The paper is called the
stationary phase
because it stays in place. The salt water is called the
mobile phase
because it is the part that climbs up the towel. The colors are called the
analyte
because they are what we are analyzing.
Paper chromatography separates compounds based on how polar they are. A
polar
molecule has one end that is more positively charged than the other end. A nonpolar molecule does not have charged ends. Paper is made of cellulose, which is a polar molecule. Salt water is also polar, but to a different extent. As the dye molecules encounter the paper and the salt water, some are bound more tightly to the paper, and some more tightly to the water. This causes the separation.
You can take advantage of this effect by using solvents that are less polar than waterâfor instance, isopropyl alcohol (rubbing alcohol) or acetone (nail polish remover)âto get different separations.
With adult supervision,
you can experiment with ¼ cup of each one to see what works best for the molecules you are trying to separate.
Using 91 percent isopropyl alcohol, the following chromatogram shows that Red #3 travels faster than the others.
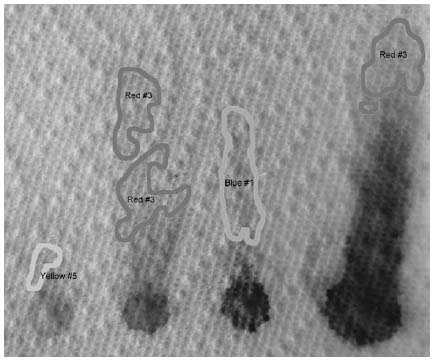
The other colors don't separate nearly as well as they did in water. In acetone (not shown), only the Red #3 moved. What this shows us is that Red #3 is the least polar of the molecules, and Blue #1 is the most polar.
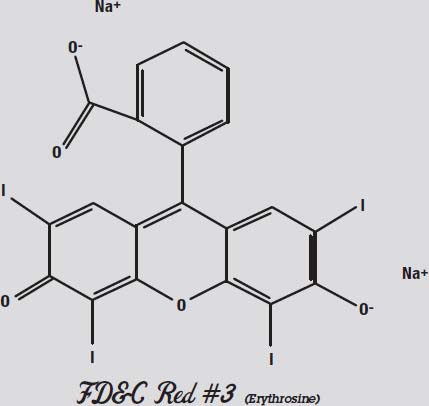
Red #3, shown above, is not very polar. Both ends have negative oxygen atoms attracting positive sodium atoms.
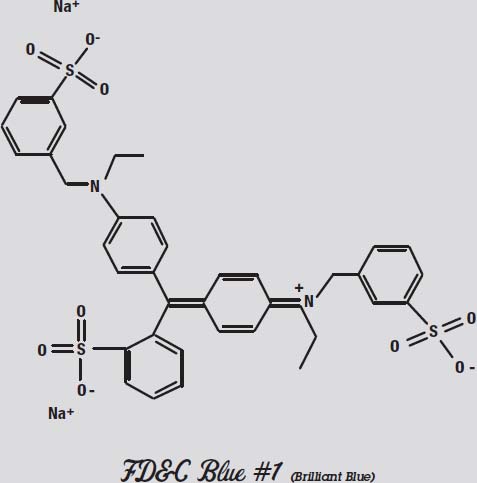
FD&C Blue #1, on the other hand, has one of its negative oxygen atoms alone at the far right, and the compensating positive nitrogen atom is buried towards the middle. This molecule is more polar than the other colors.
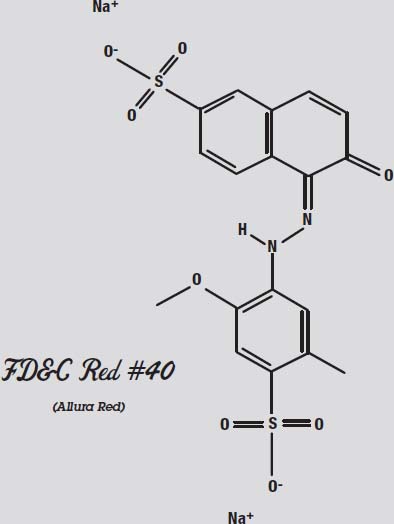
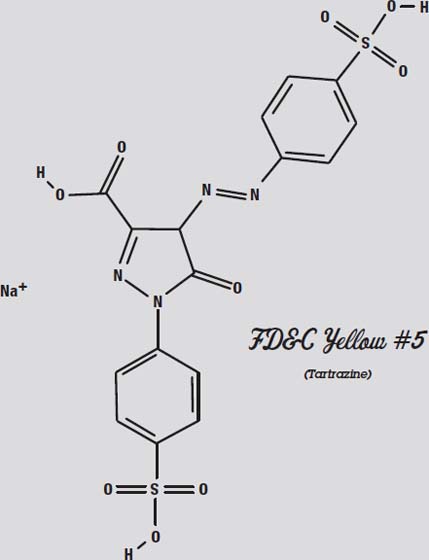
Red #40 and Yellow #5 are very similar molecules. That is why they are hard to separate. They react similarly to both the paper and the solvent.
While chromatography was originally invented to separate colored molecules, the technique is so useful that it is now used for a large number of molecules that have no color.
To make them visible, chemists can view them in ultraviolet light and look for fluorescence, or they can add a chemical to the developed chromatogram that makes the different spots visible. One such chemical is iodine. Vapors of iodine are allowed to react with the finished chromatogram, and the resulting compounds are often colored.
Other types of chromatography use sensors other than the human eye to distinguish the different molecules. This allows scientists to analyze a huge number of molecules that do not interact with visible light.