Why Is Milk White? (9 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

When these small molecules contact water, even the only very slightly dampness of dry fingers or wood, they quickly join up into long chains (polymers) that bond surfaces (such as fingers) together.
Superglue forms a strong bond with wood, plastic, and leather. It is used to lock nuts onto bolts because while it bonds well to
metal, it has a low
shear strength
, so the nut can later be removed from the bolt using a wrench.
Formulations of superglue have been made for medical use, to glue wounds together without stitches and to slow bleeding.
Cyanoacrylates react strongly with cotton, generating heat and smoke, and can in some cases cause the cotton to ignite. They also react strongly with baking soda (sodium bicarbonate). This is sometimes usefulâfor example, filling a hole with baking soda and then adding superglue can make a strong space-filling material.
Hair can be dyed using simple coloring agents, such as henna, that simply add a dye or pigment to the hair, or it can be dyed using complicated chemical reactions that open up the hair shaft, bleach the hair to a lighter color, and then react with the hair to form a permanent bond that colors the hair the desired shade.
It is not easy to make a dye stay stuck to hair. This is a good thing if you want to dye your hair blue for Halloween. Temporary dyes usually have large molecules that cannot penetrate into the hair shaft. They will wash out easily with shampoo. Since they contain no bleaches or ammonia, they are gentle on hair.
If you want to cover gray or darken light hair, slightly more permanent dyes that will last a number of washings are available. Some don't use alkaline developers to open up the hair to absorb the dye; instead they rely on the small size of the dye molecules to help penetrate into the hair. They will last through four to five shampooings.
For a little more permanence (20 or more shampooings), a gentle alkali-like sodium carbonate and a low-strength solution of hydrogen peroxide can open up the hair and chemically bind the dye to the hair. Since the low strength of the peroxide does not bleach the hair, the resulting dye looks more natural, as the variations in the hair still show through.
For the most permanent dye, one that will not wash out, ammonia and strong hydrogen peroxide are used. The peroxide bleaches the hair so that dark hair can be dyed a lighter color. But the main reason for using the peroxide is because the dye is actually formed from small molecules that bind to the hair and then react with the ammonia and peroxide to form larger molecules that are locked into the hair strand.
There are two molecules that go by the common name
ammonia.
This first is a gas, made from a nitrogen atom and three hydrogen atoms.
Ammonia gas is also known as
anhydrous ammonia,
which means ammonia without water. Ammonia reacts with water in the environment very easily, so almost all of the ammonia you will ever encounter is actually ammonium hydroxide.
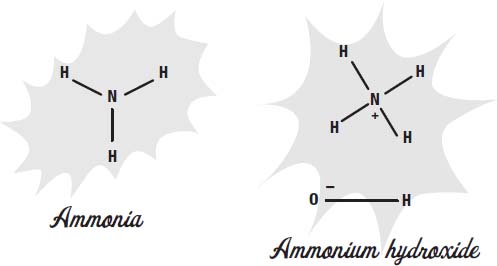
The ammonia molecule steals a hydrogen nucleus (a proton) from water. This makes the ammonium ion NH
4
+
and leaves the hydroxyl ion OH
-
as the only thing left of the water molecule. Since they have opposite charges, they attract each other and hang
around the same neighborhood. Household ammonia, used as a cleaning agent, is actually water and ammonium hydroxide.
Ammonia is a base, like lye (sodium hydroxide). Like lye, it can react with oils and fats to form soaps. As a cleaner, ammonia turns fats and oils on glass or tile surfaces into soap, and the water in the ammonia solution dissolves the soaps so the sponge or paper towel can carry them away. What is left is a solution of ammonium hydroxide, which then completely evaporates, leaving no streaks on the surface.
Animals make ammonia from proteins in the food they eat, and they use the ammonia to neutralize acids in their urine. This is why a crowded barn or stable has a strong ammonia scent.
Antifreeze is anything that prevents water from freezing when the temperature drops below the freezing point. It works by lowering the freezing point of water.
The
freezing point
is the temperature at which water freezes as fast as it melts. Below the freezing point, molecules of water move slower and will be captured by the ice. Above the freezing point, ice will melt as the molecules of water move faster.
Because heat is the random motion of molecules, there will always be some water molecules that are moving fast enough to be liquid and some that are moving slow enough to be solid ice, no matter what the temperature. But if the temperature is too warm or too cold, you may not notice, since only a tiny amount will be in the “wrong” form and only for a tiny amount of time.
The balance between melting and freezing is easy to upset. If salt is added to ice, the salt will dissolve in the water on the surface of the ice. The salt molecules (or ions) mix with the water. So now, if the liquid part is half salt molecules and half water molecules, only half as many water molecules will hit the ice as they jostle around. This means the freezing rate will be half what it used to
be. But the melting rate is unchanged, so the ice melts. The balance between freezing and melting has been upset.
To get the balance back, the temperature has to be lowered. This is why salt water freezes at a lower temperature than fresh water. Salt is thus an antifreeze.
Alcohol will do the same thing as salt. The alcohol molecules mix with the water, so fewer water molecules hit the ice. Alcohol is also an antifreeze. It is more expensive than salt, but it does not cause metals to corrode like salt does, so it is a better choice for use in a car.
An even better antifreeze than alcohol is
ethylene glycol.
If half the water is replaced with alcohol, the freezing point is lowered by 32° Celsius (57° Fahrenheit). If half the volume is ethylene glycol, the freezing point is lowered by 34° Celsius (62° Fahrenheit). This small improvement in freezing point is not as important as two other features of ethylene glycol: it is not as flammable as alcohol and it raises the boiling point of water.
If you live in a place where the temperature never drops below freezing, you don't need antifreeze. But, as mentioned in the last answer, the antifreeze ethylene glycol also raises the boiling point of water, so you might still want to add it to your radiator water so
the car doesn't boil over as easily as it would with water or a combination of alcohol and water.
Commercial antifreeze also contains rust inhibitorsâsilicates, phosphates, and boratesâto make the engine last longer. These control corrosion by keeping the liquid slightly alkaline. A green or red dye is also added so you can tell antifreeze from other liquids that might leak under your car. Orange-dyed antifreeze has rust inhibitors made from organic acids, which last longer.
Another antifreeze ingredient is diethylene glycol, although usually it is found in much smaller amounts and sometimes only because it is an unwanted byproduct of ethylene glycol production.
If you had pure iron and put it into pure water, very little would happen, since there would be no oxygen to react with the iron. And if you put the pure iron into pure dry oxygen, very little would also happen. The outer iron atoms would rust, but then that layer of rust would stand between the iron and the remaining oxygen.
Water helps iron react with oxygen. The first step in getting oxygen to react with iron is to break up the oxygen molecule. In water, oxygen can steal some electrons from iron to make four hydroxyl ions (the OH
-
ions in the following reaction):
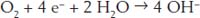
The electrons come from the iron:
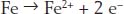
But to make rust we need another reaction with iron:
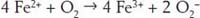
In the process of making rust, the ferrous (Fe
2
+) and ferric (Fe
3
+) ions also react with water to form Fe(OH)
2
and Fe(OH)
3
(ferrous hydroxide and ferric hydroxide) and hydrogen. These hydroxides can then lose their water to form still more iron compounds. It is all these reactions that end up making the rust flaky, so it falls off the iron and exposes new iron that can start to rust.