Why Is Milk White? (11 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

A lot of the chemicals in makeup are perfumes.
Hexyl cinnamic aldehyde is the name for the scent in oil of chamomile, and it is one of the molecules that give chamomile tea its aroma and flavor. To keep the fragrances in the makeup and to keep them on the skin, fixatives are added. One fragrance fixative is an ester of benzyl alcohol and benzoic acid called benzyl benzoate.
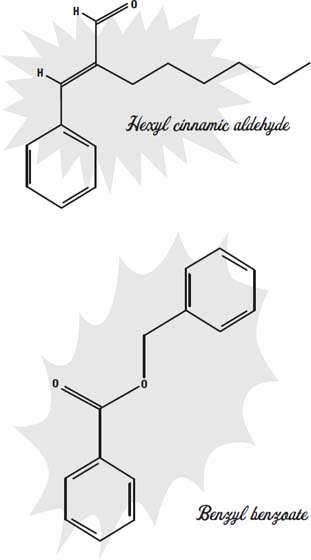
Benzyl benzoate also has the side effect of being an insecticide that kills skin mites that can cause the disease scabies.
The reason we breathe is to get oxygen into our lungs. But we also breathe to get chemicals such as carbon dioxide out of our bodies.
The lining of our nose and throat contain mucus and cilliary cells that trap and remove particles of dust in the air we breathe. These get several chances to remove particles, since the air moves past them as we breathe in and out, and the dust particles have a good chance of being collected before they actually touch the surface of the lung.
When smokers inhale cigarette smoke, it is to get the chemical nicotine into their lungs, where it then enters the bloodstream and is carried to the brain. Other molecules from smoke also enter the blood this way, such as carbon monoxide. It holds on to the hemoglobin (the protein that carries oxygen) in the blood more strongly than oxygen does, so less oxygen gets to the brain and muscles.
Other molecules in smoke get into the bloodstream or lodge in the lungs, where they can cause lung cancer or cancer of the mouth, throat, and larynx.
Some chemicals around the house are very irritating to breathe. Ammonia and bleach both contain strong alkalis that can damage the cells they contact. Some metal polishes contain acids that also damage cells.
Some chemicals can make you dizzy or cause unconsciousness. Doctors and dentists have you breathe molecules like nitrous oxide (laughing gas) or halothane to make you sleep through what would otherwise be a painfully unpleasant experience.
Shoe polish is made from waxes, oils, naphtha, turpentine, ethylene glycol, and vegetable gums. It was originally invented as a waterproofing material for leather shoes. Later, people discovered
that shoes could be given an attractive, glossy appearance with polish and buffing, and shiny shoes became fashionable.
Waxes like carnauba wax and beeswax help repel water and add shine. But to help them attach to leather, emulsifiers such as lanolin and ethylene glycol are added. These also allow the waxes, oils, and solvents to mix with water to form a thick butter-like emulsion that makes the waxy paste easier to apply to shoes. To thicken the emulsion, vegetable gums such as gum arabic are added.
Naphtha is a petroleum-based solvent that dissolves the wax to make it easier to apply. Turpentine is a similar solvent distilled from the sap of pine trees. Both of these dry quickly, allowing the waxes to harden on the surface of the leather while retaining their shiny, hard surfaces.
Shoe polish is colored by adding a form of fine carbon particles called lampblack for black polish and other dyes or pigments for other colors.
Mineral oil. When people talk about oil paints “drying,” they aren't actually talking about the same process by which a wet towel dries.
When oil paints react with oxygen in the air, they
polymerize.
That means the molecules link up into very long chains, becoming a solid but flexible plastic film. The pigment particles in the film of oil become trapped in the plastic film, which sticks to the surface it is painted on.
You would not want paint on a barn or on a canvas to stay oily and wet. But face paints are differentâthey are designed not to harden into a plastic. To do this, they are made from oils that don't oxidize in air. These oils are made from petroleum and are called mineral oils.
Face paints are mostly talcum powder mixed with mineral oil and pigments. To get them to stick to the skin, some lanolin, cetyl alcohol, triethanolamine, and some fatty acids are added, all of which have molecules where one end is attracted to oil and the
other end is attracted to water and the proteins in the skin.
These ingredients help the face paint wash off (several of them are detergents and emulsifiers), and some react with air at the surface of the paint just like regular oil paint, to make a drier surface that does not transfer easily if touched.
Pre-soak stain removers are used when clothing has a stain that we expect will be too difficult for normal laundry detergent to remove.
Normal laundry detergents often contain inexpensive
ionic surfactants
(such as sodium laureth sulfate) that work best in warm or hot water. But hot water can help set some stains. A spray-on presoak stain remover like Shout has nonionic surfactants that start to work cold, as soon as they are sprayed on.
Nonionic surfactants
work in hard water (unlike many ionic surfactants), work in acid or alkaline solutions, and have good cleaning, foaming, and emulsifying properties that can be delicately tuned by controlling how the molecule is made.
Tuning these surfactants is a matter of controlling how long the water-loving end is and how long the oil-loving end is.
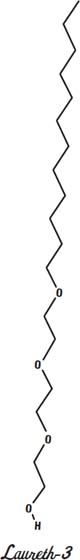
In the drawings shown on this page and the next, three different
polyethoxylated linear alcohols
are shown. These are nonionic surfactants that are designed to have different lengths of water-loving ends (the ends on the bottom). Lauryl alcohol is the base. It is the chain of 12 carbon atoms on the top of the molecule. It dissolves in fats and oils.
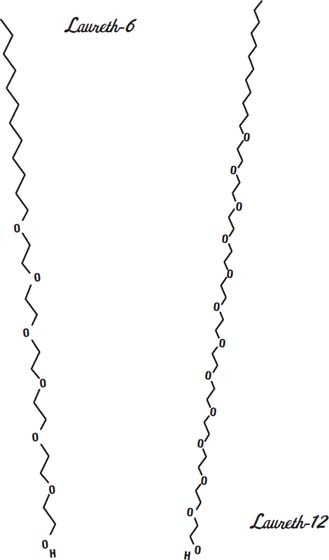
The bottom of the molecule has (in these three cases) 3, 6, or 12 molecules of ethylene oxide added. The more ethylene oxide a molecule has, the more easily it will dissolve in water.
Molecules with less than 10 ethylene oxide units dissolve mainly in fats and oils. Those with more than 10 are water soluble. Those with 7 to 11 ethylene oxides are good for making water-inoil emulsions (similar to butter or margarine). Those with 12 to 16 units are good for making oil-in-water emulsions (like mayonnaise). Those with 11 to 14 units are good for allowing water to wet fabrics easily. Those with 12 to 15 units make good detergents.
Shout uses several polyethoxylated linear alcohols for their different properties. Some smaller ones are used to lift oils from the fabric. Some larger ones are used to keep the oils in the water after they have been lifted off.
The alcohols can be linear (all in a line, as in the lauryl alcohol shown) or branched or have more complicated structure that includes cyclic molecules (loops). Linear alcohols are used where we want the detergent to break down easily in the environment. They pollute less, because bacteria can eat them easily.
Some formulas of Shout also include enzymes that help break down the proteins in blood and grass stains, and acrylic polymers to help them stay on the stain longer while in the washing machine.

Safety
Humans use a lot of chemistry to keep healthy and safe. They have disinfectants for kitchen and bathroom surfaces but also for their skin. They protect it with lotions and creams and wash off germs (and the things germs eat) to stay healthy.
People also protect themselves from the chemicals they use. After all, we are made of chemicals, and chemicals react with one another. Knowing something about how chemicals behave can keep us safe while we use them.
Because they want to keep their eyesight. Many experiments in chemistry are fairly safe. When we do experiments with food, for example, we usually don't need to wear protective eyewear. If a little salt or pepper gets in your eye, it might hurt, but the tears that come will safely wash the food away. But when we start to do experiments with chemicals such as acids, alkalis, or abrasive powders or when we heat something up, we want to protect our eyes from the hot or caustic materials.
We also wear protective gloves when we work with some chemicals or when we work with hot things. But while the skin on your hands might heal and only leave a scar, that doesn't stop you from using your hands. Damage to the eyes does not heal as easily, and you can have permanent damage to your vision.
Protective clothing is a good idea when working with most chemicals. It doesn't hurt to be safer than you need to be, and some experiments with harmless chemicals might produce things that are harmful. Keeping long hair away from flames is a good ideaâtie your hair back so it doesn't fall into the flame.
Most protection is common sense. We wear gloves when using strong household cleansers like bleach. We wear dust masks when using sandpaper on plaster walls or when spraying paint. We wear eye protection whenever there is a danger of flying particles or droplets getting into our eyes.
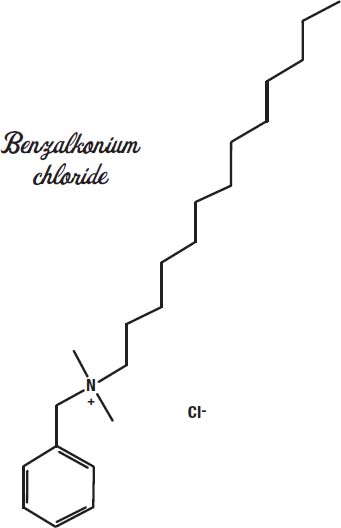
Actually, Bactine is designed not to sting. It has lidocaine in it, similar to the novocaine the dentist uses to numb teeth. The germkilling ingredient is benzalkonium chloride.
This disinfectant is used in many other products, such as Lysol, antiseptic towelettes, and newer non-alcohol-based hand sanitizers. It works by disrupting the cell walls of bacteria and disabling their enzymes, due to its action as a surfactant.
As a replacement for alcohol and hydrogen peroxide, benzalkonium chloride is used because it irritates the wound less. It is even used as a preservative in some eye drops and nasal sprays. Alcohol kills germs by drying them out, and other disinfectants (hydrogen peroxide, iodine, chlorhexidine) act by oxidizing (burning) germs. Both of those actions also harm skin cells and can cause stinging.
Lidocaine is used as the local anesthetic in Bactine because it acts very quickly. When the disinfectant is first sprayed on the cut
or scrape, the temperature difference and the initial contact can stimulate pain nerves. But the lidocaine should quickly quiet them down.