Why Is Milk White? (12 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Your skin has a natural protective layer of oil that keeps it from drying out and keeps it flexible. If there are bacteria on top of this layer of natural oil, washing with soap will remove both the oil and the bacteria that sits on top.
Soap can also kill bacteria, by opening holes in the bacterial membrane, which is made of surfactants itself, like soap is. The surfactants in the bacterial cell wall are in two layers, with all of the oil-loving ends of the molecules facing inward between the two layers. Adding soap and scrubbing allows the molecules in the two layers to turn inside-out, and this allows the bacteria to leak and soap to get inside, where it can harm the bacterium's enzymes that it needs to live.
Many bacteria stick to surfaces by creating what is called a
biofilm.
This is a film of polymers that act as a glue or slime to hold the bacteria together and help them stick to a surface.
Soap can interfere with biofilms in several ways. It can attach to the molecules that make up the slime and allow it to be washed away, it can disrupt the attachment between the biofilm and the surface, or it can interfere with the attachment of the bacteria to the film.
Once bacteria have been scrubbed off the skin, the soap attaches to the walls of the bacteria and prevents them from attaching to the skin or one another. They then wash down the drain with the rinse water.
Some hand soaps also contain antibacterial chemicals such as triclosan or hexachlorophene that kill bacteria through other mechanisms. The combination of soap and antibacterial agents is better than either one alone.
and â¦
Why can't you swallow toothpaste when you can swallow a mint?
It is not recommended that you swallow toothpaste. This is why children under six are not supposed to use regular toothpaste.
The main bioactive ingredient in toothpaste is sodium or tin fluoride. A tube of toothpaste contains enough fluoride to kill a six-year-old, and since toothpastes can come in flavors like bubble
gum or watermelon, they should be kept away from young children. That said, few problems are actually reported, and the problems have not been fatal, even when severe.
Fluorides can damage the lining of the stomach and usually cause vomiting, whereby most of the toothpaste is eliminated and not absorbed into the bloodstream.
Toothpaste made for young children does not contain fluoride and is safe to swallow. Since their teeth are temporary anyway, the lack of fluoride is not a long-term health problem.
If the pool water is properly maintained, they shouldn't. People add chemicals to swimming pool water to control microbes such as bacteria and algae. Chlorine can irritate eyes, but when that happens it's usually an indication that too much was used. A chemical called cyanuric acid is added to pool water to protect pool chlorine from sunlight. Cyanuric acid forms a weak bond with free chlorine in the pool water, preventing it from evaporating out of the pool. It also keeps free chlorine from irritating your eyes.
If there is too little chlorine in the pool water, this can cause cloudiness and the production of chloramines (that is what smells like chlorine to your nose). These can cause eye irritation.
Other things that can irritate the eyes are pH imbalances and hard water. We add acid or alkali to swimming pool water to adjust the pH to a neutral level. If this is done properly and often enough, eye irritation due to acidic or alkaline water will not be a problem.
There are a number of mechanisms. First, just wiping off excess oil using a clean paper towel can help prevent acne. So the face wipes can help even if they have no other ingredients at all. Face wipes also include detergents that make the oils wash away easily. Some use alcohol to both remove oil and kill germs.
But the three main acne-fighting ingredients are salicylic acid, benzoyl peroxide, and benzalkonium chloride.
Benzalkonium chloride was discussed with Bactine earlier (
page 80
). It kills germs by breaking up their cell walls and disabling their enzymes.
Salicylic acid works by getting inside the hair follicles (the clumps of cells in the pore that create hair) and removing dead cells that can clog the pores. It causes the dead cells to swell up with water, helping push them out. It is thus useful for preventing acne in the first place and less useful for treating pimples that are already inflamed.
Benzoyl peroxide kills the primary type of acne-forming bacteria,
Propionibacterium acnes.
It is thus the opposite of salicylic acid, in that it is good for inflamed pimples and doesn't do much for blackheads and whiteheads. Benzoyl peroxide kills germs by breaking down and releasing free oxygen, which burns the bacterial proteins. Bacteria cannot develop resistance to this type of antibacterial agent.
Benzoyl peroxide may also act as an irritant, causing the cells in the pores to grow faster and slough off faster, pushing out the waxy sebum that plugs up the pore. Because benzoyl peroxide dissolves in fats, it can penetrate into the sebum and get into the pore, where water-based medicines cannot.
Zanfel is a cream that contains several strong surfactants (detergents) and tiny polyethylene beads. The main detergent in Zanfel is sodium lauroyl sarcosinate.
This detergent has a capability we have not discussed in other detergents. It is a
penetration enhancer.
That means it helps other molecules penetrate deeper into the skin. It is an ionic surfactant (you can see the plus sign by the sodium ion and the minus sign by the oxygen). It is derived from coconut oil and cleans without completely stripping the skin of all oils.
The second detergent in Zanfel is nonoxynol-9.
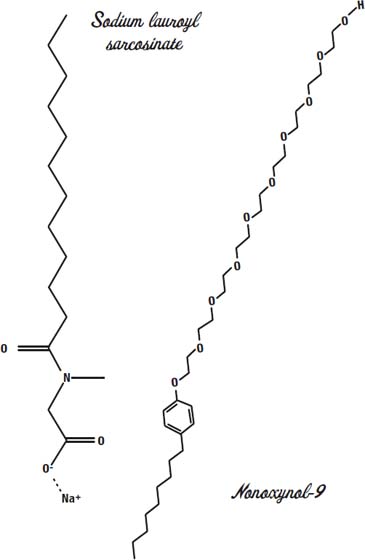
Nonoxynol-9 is a nonionic detergent. We saw these earlier when we discussed laureth-12 in Shout stain remover (
page 75
). The long chain of nine ethylene oxide groups is water-loving, and the long fatty acid chain at the bottom is oil-loving.
In the section on another surfactant, benzalkonium chloride, the antiseptic in Bactine (
page 80
), the discussion mentioned that some surfactants are good at breaking up cell walls and killing microbes. The same thing happens with nonoxynol-9: it is used in Zanfel to break up the poison
urushiol
that causes poison ivy and poison oak rashes.
Nonoxynol-9 is a polyethylene glycol. The long chain with all ethylene oxide units (two carbons and an oxygen) is the polyethylene part
(poly
means “many”). Another polyethylene glycol is C12- 15 Pareth-9, and it is the third detergent in Zanfel.
To make these detergents work better in hard water, sodium EDTA is added. It grabs onto magnesium and calcium ions in the water and keeps them from interfering with the detergents. To keep the detergents from spoiling, a
bactericide
(bacteria killer) called quaternium-15 is used. It releases formaldehyde to kill germs.
Another surfactant, triethanolamine (which is three ethylene molecules all attached to a central nitrogen atom) helps make the urushiol soluble in water. It also adjusts the acidity of the product and neutralizes fatty acids.
There are actually two different types of products that protect against sunburn: sunblock and sunscreen.
Sunscreen
absorbs ultraviolet light and turns it into heat. Molecules that absorb particular wavelengths of light are called dyes. A sunscreen is a dye that absorbs the invisible ultraviolet light that causes sunburn.
One such dye molecule is benzophenone-3. It blocks primarily the UVB light that causes sunburn but not much of the UVA light that can cause skin cancer.
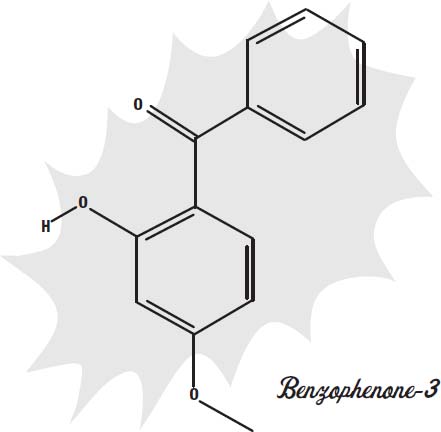
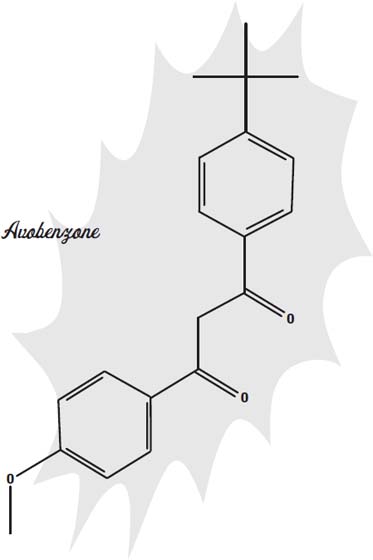
Another sunscreen, avobenzone, is better at blocking both forms of ultraviolet light. There are at least 23 other similar compounds that act in much the same way.
A different type of protection is offered by
sunblock.
In a sunblock, the ultraviolet light is reflected instead of being absorbed. In some sunblocks, almost all light is reflected, including visible light, so the product looks bright white. In others, the size of the reflecting particles in the cream is carefully selected so that visible light is not reflected and the skin colors are not blocked.
Sunblocks typically include the same pigments used in white paint, specifically titanium dioxide and zinc oxide. Zinc oxide is
better at blocking the entire ultraviolet spectrum than titanium dioxide.
You may have noticed that on a cloudy day you can still see things. The sky is not totally dark, like it is at night. This shows us that
some light is getting through the clouds. That light includes ultraviolet light that can cause sunburn and skin cancer.
Cloudy days are usually cooler, so the skin does not feel warm and there is less of an urge to get into the shade or go indoors to cool off. So you might actually stay outdoors for a longer period. If you are at the beach on a cloudy day, you may be wearing less clothing, allowing more light to get to your skin.
Also, on cloudy days, you may not remember to use sunscreen.
You get a tan by damaging your skin. People need sunlight to produce vitamin D. As humans moved from Africa into northern countries that did not get enough sunlight to produce adequate vitamin D, they evolved to produce less of the protective melanin pigment that blocks ultraviolet radiation and the damage it can cause.
Instead of producing melanin pigment in their skin all the time, light-skinned people only produce a little, unless the skin detects damage from ultraviolet light. Then it starts producing more melanin. It takes about three days for the melanin to be produced, so if there is a lot of sun exposure before the tan is produced, you will get sunburned.
When you tan, you get a little bit of tanning effect right away, because the longer wavelength UVA light causes damage to what melanin exists, causing it to combine with oxygen. This makes it darker. But most of the tan comes from increased production of melanin, which takes longer.
The color of your skin without melanin can be seen inside your mouth. The pink color is due to the color of the blood flowing in the fine capillaries (tiny blood vessels) and the scattering of light by the skin cells, which would look white if it were not for the red blood. The red and the white combine to look pink.
Ultraviolet light damages skin in several ways. It breaks up DNA, which is why it can cause cancer. It is the products of DNA
destruction that trigger the tanning process, so you can't get a tan without first damaging your skin and risking cancer. But the sun also damages the proteins that make skin flexible. This results in dry and wrinkled skin, an effect called
premature aging
, since it makes people who are in the sun a lot look much older than people who get less sun.