Why Is Milk White? (14 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Mostly water. About 98 percent water, in fact. Saliva also contains dissolved salts, mucus, some antibacterial molecules, and enzymes such as amylase, which breaks down starch into simple sugars. Another enzyme, lipase, breaks down fat. So saliva is actually the first step in the digestive process.
Antibacterial agents such as peroxidase, immunoglobulins, lysozyme (an enzyme that breaks open bacterial cell walls), and lactoferrin (a protein that kills bacteria and fungi) are produced in saliva.
Saliva in mice includes a hormone called nerve growth factor, which helps healing when they lick their wounds. It is not a component of human saliva, however.
The cells in your mouth and nose do not have the tough protective barrier that skin on the outside of the body has. They are instead protected by mucus. Anywhere we don't have skin there is a layer of mucus that protects the cells from bacteria, fungi, and viruses.
Mucus is water made thick and sticky by the addition of proteins that have simple sugars attached to them. These are called glycoproteins. They help the mucus stick to the walls of the mouth and nose, and they trap bacteria and particles so that they don't get into your lungs. The lungs also have a mucus layer to deal with anything that gets past the mouth and nose.
Mucus lubricates the food we eat, so it is easier to chew and swallow. When you see, smell, or even think about food, your salivary glands start to produce fluids in anticipation of the need to moisten and lubricate the food you are about to eat.
The cells that line your nose and throat contain cells that constantly move mucus toward the back of the throat, where it can be swallowed, along with any particles and bacteria it has trapped.
Besides trapping bacteria, mucus contains enzymes that break open bacterial walls. The enzyme lysozyme and the protein
lactoferrin are part of what is called the
innate immune system.
This is the system that works against any bacteria that comes along. The
adaptive immune system
recognizes germs so we don't get the same disease again.
Lactoferrin has oxidized iron (rust) in it that makes it look red when purified (but the amount in saliva is so small it does not have a color). Lactoferrin binds to bacterial cell walls, and the oxidized iron produces peroxides that break down the walls and cause the bacteria to leak.
Lactoferrin attracts white blood cells to bacteria, so they get eaten up. Its main function elsewhere in the body is to ferry iron around to where it is needed. But it has many other functions. In saliva, it binds iron that bacteria need to live so it is not available to them, it binds to the cell walls, it intrudes inside the bacterium, it releases peroxides, and it interferes with the enzymes in the bacteria or fungus.
Lactoferrin binds to the same sites on cells that viruses do, so it blocks viruses from entering cells. It also binds directly to some viruses, and it can prevent viruses from growing inside cells, by attracting natural killer cells and macrophages to come along and kill the infected cell.
Chlorine bleach is the first one that comes to mind. If an acid like vinegar is added to it, it produces poisonous and irritating gases such as chlorine and hydrogen chloride (which makes hydrochloric acid when it contacts the water in your eyes, throat, and nose).
Mixing bleach with ammonia can produce poisonous chloramine vapors.
Most of the strong chemicals in your house should not be mixed, if only because it makes them less effective. Oftentimes when strong chemicals are mixed together, they generate a lot of heat and can boil, splattering caustic chemicals onto your skin or
into your eyes. Lye and drain cleaners have to be very carefully handled for this reason. They can boil the water they are added to.
Pesticides should never be mixed with anything other than what the label recommends for diluting them. They are poisons to start with, and causing a chemical reaction can liberate poisonous vapors or gases that you might not even be able to smell, so you might not know you are poisoning yourself.

Fire or Go Bang
Most people delight in fire and explosions of all kindsâfireworks, cap guns, birthday candles, sparklersâand an action movie without explosions is hard to find. I like to use this fascination, this “teaching moment,” to explain the science behind the pyrotechnics. Each time a question is answered, a new questions pops up, and the budding scientist gains new understanding of her world.
An explosion happens when something burns so fast that it makes a bang. We usually see things burn in the open, where wood, paper, or some other burnable material gets the oxygen it needs from the air, just like we do. But an explosive carries its own oxygen in the compound. It keeps the oxygen right next to the ingredients that burn, so the burning can happen very quickly.
Some explosives are very easy to set off. The explosives in cap guns can be set off by just hitting them with the tiny hammer in the gun. Gunpowder can be set off with a match. Nitroglycerin will explode if you drop it on the floor.
To make explosives safer to use, it helps if they are harder to set off, so they only explode when we want them to. To make nitroglycerin safer to use, Alfred Nobel figured out how to let it soak into powdered clay. The result is dynamite, which won't explode if you just drop it on the floor. Inside a stick of dynamite is a little firecracker. When you light the fuse of the firecracker and the firecracker blows up, it has enough force to make the nitroglycerin explode.
There are many types of explosives in use today, and most of them are designed to be hard to set off. You can hit TNT with a hammer and it won't explode. Like dynamite, it needs a small explosive that is easier to ignite. This
primer
or
blasting cap
is often protected in a metal casing and triggered by electricity. This allows the explosive to be set off from a distance, using long wires or a timer.
To make an explosive, you need to mix something that will burn (called the
fuel),
such as charcoal or sulfur, with something that provides oxygen (called the
oxidizer).
The closer you can get the fuel to the oxidizer, the faster it will burn.
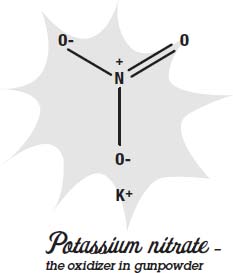
One of the first explosives to be invented was gunpowder. The fuel is charcoal and sulfur. The oxidizer is potassium nitrate, which is a chemical that has three oxygen atoms in it. When it gets hot, it releases the oxygen, so the charcoal and the sulfur can burn.
To make sure the oxidizer and the fuel are very close to each other, each of them is ground into a fine powder, and then the powder is carefully mixed together. The finer you grind the powders, the better the gunpowder will be.
To keep the powders from separating, they are mixed with a
little water to make a paste and then dried out again, after which the dried paste is ground up. This makes the gunpowder more reliable and helps it all burn up at once.
Another way to make an explosion is to mix a fuel really well with air and then ignite it. Dust explosions happen when coal dust in a mine or flour dust in a mill are mixed into the air and then set off by a spark or a flame. Gas explosions happen in much the same way, when a flammable gas or vapor mixes very well with air and then gets ignited somehow.
Nitroglycerin and TNT are examples of what are called
high explosives.
In a high explosive, the fuel and oxidizer are packed into the same molecule. They are extremely close together. That makes nitroglycerin and TNT much more powerful than gunpowder.
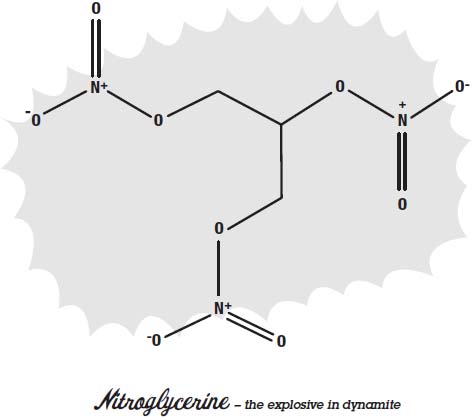
When a high explosive detonates, the molecule comes apart and the atoms rearrange themselves. The oxygen combines with the fuel very quickly, and there is a big bang.
Not all explosives use oxygen as the oxidizer, however. Other elements can act like oxygen to burn fuels. Chlorine, fluorine, iodine, and bromine are all good oxidizers.
Gunpowder that is made from charcoal, sulfur, and potassium nitrate is called
black powder.
It is not used much anymore, because it makes a lot of smoke when it explodes. It also leaves a chemical residue in the gun barrel that makes it corrode.
To solve these problems,
smokeless powder
was invented. High explosives like guncotton (cellulose nitrate) were too powerful to use in guns and cannons. However, they could be diluted with alcohol or similar liquids to make a jelly that would harden and could be cut into tiny pieces. The result was a less powerful explosive that did not blow up the gun barrels. It was still three times as powerful as black powder, and it made very little smoke.
Alfred Nobel, who invented dynamite, also invented a smokeless powder called
Ballistite,
made from camphor, guncotton, and nitroglycerin. Later, a similar mixture of nitroglycerin, guncotton, and petroleum jelly was made, and named
Cordite
because it was made into grains by forcing it out of little holes so it looked like string (or cord). The camphor and petroleum jelly slow down the rate of burning, so the powder is not so strong that it destroys the gun barrel.
Modern
propellants
are still called gunpowder, even though they aren't powders. They are little balls, flakes, or rods, and they are often coated with graphite. Graphite is a gray powder form of carbon, and it conducts electricity. Conducting electricity is important, since it prevents static electricity from building up. A spark of static electricity could cause the gunpowder to ignite unexpectedly.
The chemistry used in guns is not limited to gunpowder. In order to make the gunpowder explode, a
primer
is needed. A primer is an explosive that will detonate when it is hit by the firing pin in the gun.
You may have seen “strike anywhere” matches, which burst into flame with just a little friction. They can also be lit by hitting them with a small hammer. They are made from sulfur and phosphorus, with an oxidizer that is more powerful than potassium nitrate, such as potassium chlorate. A mixture like that could be used as a primer in a bullet cartridge. But some specialized
contact explosives
âexplosives that are easy to set offâhave been invented for just this purpose.
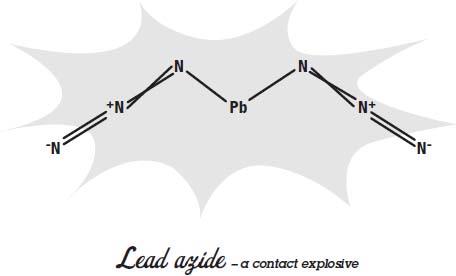
One of the early contact explosives used in primers was mercury fulminate. It is not as corrosive as contact explosives made with potassium chlorate, so it did less damage to the gun.
Other contact explosives have replaced mercury fulminate as primers in modern weapons. Lead azide, lead styphnate, and a class of compounds called tetrazenes are now used. All of these contact explosives are molecules that come apart very easily and release a lot of energy very quickly when they come apart and the atoms rearrange themselves.