Why Is Milk White? (17 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

N-heptane is just a chain of 7 carbon atoms, with 2 hydrogen atoms attached to each carbon and another hydrogen at each end. It is a component of gasoline and smells like it.
Model airplane cement uses toluene as a solvent. It also has a characteristic odor.
Some glues, like epoxy, come in two parts, which react together to form a new molecule. Each part contains some volatile molecules that have characteristic odors, but as they cure they can also emit new molecules with different odors.
Silicone rubber adhesives combine with the moisture in the air to cure, and in the curing process they release lactic acid, which has a sharp, eye-stinging odor.
There are thousands of chemicals that are used in perfumes. But many of them can be grouped according to their chemical properties.
One such group is esters. An ester is what you get when you let an alcohol react with certain types of acids (acids that contain oxygen). Esters are the main thing you smell when you sniff fruits and flowers.
The scent of pineapple, for example, is largely due to the molecules allyl hexanoate, butyl butyrate, ethyl butyrate, methyl butyrate, pentyl butyrate, and pentyl hexanoate.
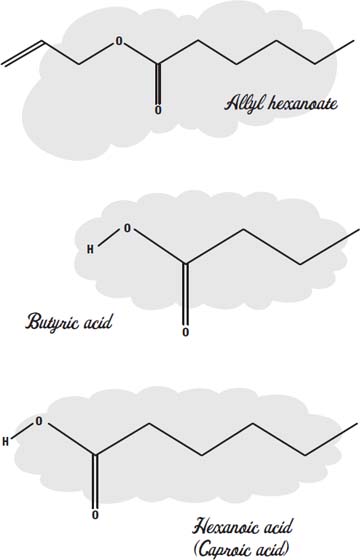
Esters that make up perfumes and sweet fruity flavorings start out with bad-smelling organic acids, like butyric acid (which gives rancid butter its odor), acetic acid (which gives vinegar its sharp smell), or hexanoic acid, which is also called caproic acid, because it is the smell of goats in a barnyard.
These foul-smelling acids combine with alcohols like methanol or ethanol (which have almost no odor) or butanol (which has a creamy, buttery taste and smell and is used as a flavoring in baked goods).
The results are pleasant-smelling floral or fruity scents used in perfumes, candies, candles, and desserts.
Another class of chemicals used in perfumes are aldehydes. Cinnamaldehyde gives cinnamon its aroma. Furfural is an aldehyde that smells like almonds. Benzaldehyde is another aldehyde in almond scent. Vanillin is an aldehyde that gives another popular flavoring its aroma.
Alcohols themselves are another group. Geraniol is found in roses, lemon oil, and (of course) in geraniums.
Ketones are another group. Civetone is the main scent molecule in musk oil. Damascone is a ketone in rose scent.
A perfume typically has a hundred or so different chemicals in it, and some have several hundred. There are about ten different odor molecules in jasmine and between three and a dozen in a rose, depending on the variety.
Plants make molecules that have aromas for several reasons. Some of the molecules are used by the plant to attract insects for pollination. Other molecules are there to repel insects that might otherwise dine on the plant.
Sometimes a plant has an odor simply because some of the molecules in it are volatile and make their way through the air to your nose. Pine scents contain many light molecules that float through the air easily. Some of these are insecticide molecules, and our noses are pretty good at detecting molecules that might be toxic.
The smell of freshly cut grass is due in part to molecules the plant gives off when under attack by insects. These “green leaf volatiles” attract predatory insects that eat the bugs that are eating the grass. In order to do their job, they have to travel through the air and land on the scent-detecting cells on the antennae of the predatory bugs. But we can smell them too.
Some of the molecules in cut grass are antimicrobials, and they are part of the plant's reaction to injury. They are there to kill bacteria and fungus. Our noses are sensitive to molecules that are active enough to kill germs.

People use their sense of sight more than any other sense. A dog may experience the world mostly through its nose, and a bat through its ears, but most humans depend on sight.
A black and white world would seem quite dull to us. We would see shapes and detect movement, both very important things, but we evolved color vision because it gives us many advantages, telling us what is ripe, what is rotten, and what is hiding in the grass.
There are actually only three colors that we can see. Our eyes contain color-sensing cells called
cones.
There are three types, and they each can sense one color. The colors are red, green, and blue.
But the cones in your eyes respond rather broadly to the light that comes in, so that some red light will also stimulate the green cones, and to a lesser extent the blue cones. Some blue light will stimulate the green cones, and the red cones a little bit less. And some green light will stimulate the blue and red cones.
As a result, colors of light that lie in between the colors that the cones receive best will stimulate two or more cones at the same time. You see light that stimulates red and green cones at the same time as yellow. Violet light stimulates the blue and the red cones.
You have seen toys that glow in the dark, and you have seen fluorescent colors in clothing and highlighting markers that seem to glow even when it isn't dark. These objects glow because they contain compounds called
phosphors.
A phosphor absorbs light of one color, and that energy is stored in the molecule (scientists say that the molecule is
excited),
and a little bit later the energy is released again as light and heat. Since some of the energy is lost as heat, the light that comes out has less energy than the light that went in. Light with less energy is redder in color.
So, to make a fluorescent light, you want to start with light that has a lot of energy (light that has a shorter wavelength). If light has a lot of energy, it will move so far in the blue direction that it goes past violet into the ultraviolet, which we cannot see.
A fluorescent bulb is a tube of glass that has a little bit of mercury in it and very little else (almost a vacuum). When electricity is put into the mercury vapor, the mercury gets excited and emits ultraviolet light, as well as some green and blue light and a little bit of red.
Now, if the inside of the tube is coated with glow-in-the-dark phosphors that absorb the ultraviolet light, they will emit exactly the colors we want. Little bits of each color of phosphor can added to tune the light to be any shade of white (or any other color) desired. The tube can simulate sunlight or get a bluer or redder light to suit the mood.
Since the mercury vapor emits mostly light and very little heat, compared to heating up a tungsten filament until it glows white hot, fluorescent lights use less energy than incandescent lights of the same brightness.
Since nail polish is a cosmetic, the pigments and dyes are selected from the list of D&C colors regulated by governments. These colors were discussed earlier in the question about the colors in shampoo (
page 31
).
The pigments in nail polish are generally dissolved with a filmforming plastic in a solvent. A typical film-forming plastic used is the explosive nitrocellulose, one of the ingredients in smokeless gunpowder. This is dissolved in a relatively safe solvent, such as ethyl acetate or butyl acetate (or both) and sometimes isopropanol.
To make the colors opaque, pigments such as titanium dioxide may be added. Thickeners such as stearalkonium bentonite (a type of clay) are also added. To get pearlescent effects, flakes of fish scales, tiny flakes of aluminum, or even minute flakes of stainless steel can be added. These give metallic sheen to the nail polish. Platelets of mica can also be used to give a shiny effect.
and â¦
Why is milk white?
and â¦
Why are clouds white?
All of these things are white because the light that hits them is scattered by all the tiny particles or droplets in them. When light hits a tiny particle or droplet of water or fat, it bounces off. All colors bounce off equally. When we see something that emits or reflects all colors at the same time, it appears white.
The fat droplets that make milk white come in a variety of sizes but group into three main diameters: 120 nanometers, 400 nanometers, and 1,500 nanometers. Visible light waves range from 400 nanometers to 800 nanometers, so the size of the fat droplets is just about the size of a wavelength of visible light.
The water droplets in clouds range from 6,000 to 14,000 nanometers. This is still much too small to see, especially when the clouds are a mile above you, so we see what looks like a solid object.
If particles are too small, a powder does not look white, especially when it is in a liquid. This is why the same chemical, titanium
dioxide, can be a bright white in paint but invisible in sunscreen if the particles are smaller than a wavelength of visible light.
The sky is actually a little bit on the violet side. It only looks blue because your eyes are much more sensitive to blue light than to violet light.
When white light from the sun travels through clear air, it hits the molecules of nitrogen and oxygen and gets scattered a little bit, so it travels in a slightly different direction. Since there are miles of air between you and the sun, the light will scatter many times.
But how much the light is scattered depends on the color. Blue light is scattered about 10 times more than red light. This is called
Rayleigh scattering,
after the man who worked out the math. Violet light is the light that is scattered the most out of all the light your eyes can see. This is why the sun looks yellow to you: white light from the sun will look yellow if you remove the violet light by scattering it away.
But your eyes are not very sensitive to violet light. They are very sensitive to blue light. They are also sensitive to green light and red light. A little bit of the violet light excites the red light sensing cones in your eyes, which is why violet looks like blue with a little red in it. It is also why the sky looks light blue instead of deep blue.