Why Is Milk White? (20 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

At the copper strip, the extra electrons attract the hydrogen ions in the solution to migrate toward the copper. When they get there, they can steal the electrons to become hydrogen gas. The hydrogen gas bubbles up out of the solution. This keeps up until there is no more aluminum left on the aluminum strip. All the electrons flowing from the aluminum to the copper have been moving the needle of the meter, showing us that electricity has been produced.
In some ways, solar panels work like batteries and thermocouples. Two dissimilar conductors are placed together, and electrons move from the one that holds them loosely to the one that holds them tighter.
There are many types of solar cell materials, and not all of them work in the same way. In the simplest ones, a photon of light knocks an electron off a metal, and that electron flies off to another conductor and then goes through a circuit to get back to the original metal plate.
But most solar cells these days use semiconductor materials in what is called a
p-n junction.
In a silicon solar cell, the two sides of the p-n junction are made of silicon. A small amount of phosphorus is added to one side to make the n material. A small amount of boron is added to the other side to make the p material.
Phosphorus has one more outer electron than silicon does. Boron has one less. The electron from the phosphorus becomes a conduction electron, allowing electric current to flow in the material. In the p material, the missing electron from the boron creates a “hole.” An electron from silicon can move into the hole, leaving another hole behind. In this way, the holes can appear to move around.
When the p and n materials are put together to form a p-n junction, the extra phosphorus electrons in the n material are attracted to the holes left by the boron in the p material. This creates a
voltage gradient
in the cellâa gradual change in the voltage that causes electrons to slide down it. (Think of it like a playground slide, which is a gravity gradient that causes
you
to slide down it.)
When a photon of light hits the semiconductor, an electron is excited and becomes a conduction electron. It moves along the voltage gradient from the negative side of the cell to the positive side. The hole it created when it left the silicon atom moves toward the positive side, just as a bubble of air in water floats to the surface.
When the solar cell is connected to a meter or to a light bulb, the electrons can flow through the wires to get back to the other side of the p-n junction, filling in the holes, so the whole process can start over again.
A Van de Graaff generator is a device for making very high-voltage electricity. It usually has a metal sphere situated above a plastic tube. Inside the tube is a rubber band that is made to move by a motor. A wire brush is attached to the metal sphere and almost touches the rubber band. Another wire brush almost touches the rubber band at the bottom.
Rubber is an insulator, so electrons on it cannot easily move around. This means that when a few extra electrons are added or a few are taken away, the negative or positive charge remains in that place on the rubber band.
The rubber is attached at the top to a pulley made of glass, Teflon, or some other material. As the rubber leaves the glass pulley, electrons are transferred from the glass to the rubber, because rubber holds electrons more tightly than glass does.
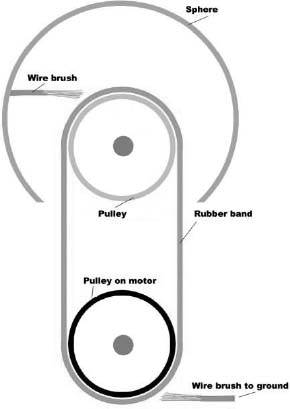
The glass is now positively charged. It pulls electrons from the wire brush at the top. This leaves the metal sphere with a positive charge. The positive charges repel one another and flow to the outside of the sphere, leaving the inside uncharged, so it can always lose electrons.
As the rubber band moves around, the negatively charged rubber is moved down next to the bottom wire brush. The electrons jump onto air molecules and then onto the wire, always trying to get away from one another.
The rubber is now neutral and is brought around to the glass pulley to start the whole process over again. The top sphere becomes more positively charged with each revolution of the rubber band. The electrons leave by the bottom wire, where they build up on any surface they are connected to. Eventually, the charge separation (voltage) is so high that electrons are stolen from the air, and the air becomes conductive. A big spark conducts electrons to the sphere, and the voltage drops again.
Then the whole process starts over.
Most of chemistry deals with water, and water has been called the “universal solvent.” Both of these things have to do with water's ability to form
hydrogen bonds.
These bonds are the reason sodium chloride can dissolve easily in water. In a molecule of water, the oxygen atom attracts the electrons from the hydrogens so strongly that they stay around the oxygen atom most of the time and only sometimes swing back around to the hydrogen atoms. This makes the oxygen atom slightly negative and leaves the hydrogen atoms slightly positive. The positive hydrogen atoms on the water molecule attract the negative chloride ions in the salt. The negative oxygen atom in the water molecule attracts the positive sodium ion in the salt. This attraction competes with the attraction of the sodium and chlorine for each other and has the effect of weakening their attraction. If there is enough heat energy to jostle the atoms around, the salt will dissolve in the water.
More generally, a hydrogen bond is the attraction of the positive hydrogen nucleus to negative ions or negative parts of other molecules. Hydrogen bonds also make the water molecules attract
one another,
so water is a liquid at room temperature. Without hydrogen bonds, it would be a gas, like it is when it is hot enough that the motion of the molecules overcomes the hydrogen bonds.
Hydrogen bonds are what make ice take up more room than liquid water, so ice floats. When water freezes, hydrogen bonds lock the molecules into place, and the shape of the water molecule forces them into hexagons that take up more room than the randomly moving water molecules in liquid water. Hydrogen bonds are also very important in shaping proteins in living things, and water affects how the proteins work.
An atom is the smallest thing a chemical element can be divided into. An atom can be broken down into smaller parts, but then it would no longer be the same chemical element.
An atom is made up of a nucleus around which are some number of electrons. The nucleus is made up of a number of protons, whose positive charges attract a similar number of electrons, which have negative charges. The number of protons an atom has is its atomic number and determines what chemical element the atom is.
Hydrogen is an element with a single proton. It attracts a single electron. That electron can be taken away, leaving a hydrogen ion. An ion is an atom that is either missing one or more electrons or which has one or more extra electrons.
Hydrogen can also have one or two neutrons in the nucleus, along with the proton. Neutrons don't change the atomic number (the number of protons), so the atom is still a hydrogen atom. But they change its weight, so an atom with a proton and a neutron is called heavy hydrogen or, more commonly, deuterium. A hydrogen atom with two neutrons is called tritium.
Chemistry deals mostly with the effects of protons and electrons. The weight of an atom affects how it reacts with other atoms, but to a far smaller degree than its atomic number.
Atoms are extremely small. The nucleus of an atom is even smallerâso much so that an atom is mostly empty space. The electrons in the atoms are negatively charged, so they repel one another, and they also repel the electrons in other atoms. The reason you don't fall through your chair is that the electrons in your pants repel the electrons in the chair. But the atoms in your pants and the chair are still mostly empty space.
Everything is a chemical. All of what we call things are made of atoms. A chemical is just something made of atoms.
But to stop there would be to ignore the common use of the term
chemical
, which is used to mean something made by chemists, as opposed to something found in nature. So while water is a chemical and vodka is a mixture of chemicals, neither is considered a “chemical” by people who lack an understanding of chemistry. Rubbing alcohol, a mixture very much like vodka but even more poisonous, is generally considered to be a chemical by such people, even if the distinctions are hard to understand.
So gasoline may or may not be considered a chemical by people who don't understand chemistry. But the additives that are in the gasoline to make it work better are all considered chemicals.
To many people, chemicals are anything listed in an ingredients label that they don't understand or that smells unusual. The acid that they put into their swimming pools is a chemical, but the same acid they produce in their stomachs to digest food is not.
So the things in everyday life that are considered chemicals are generally those substances used to clean, to disinfect, to prevent spoilage, to color, to glue, or to medicate.
Sometimes whether something is called a chemical depends on how it is used. If ethanol is in paint thinner, it is a chemical. If the same ethanol is in vodka, it is not a chemical. If sodium chloride is in your shampoo, it is a chemical. On your potato chips, it is not.
Elements
are the smallest, simplest things a chemist works with. Everything else is built from elements.
Physics deals with things smaller than atoms. Chemistry deals with atoms. Even when chemists talk about smaller things, like protons and electrons, it is how they affect atoms or interact with atoms that the chemist is concerned about. The periodic table of the elements is in one sense just a list of all the building blocks that chemists play with. It starts with the smallest atom, hydrogen, and
goes on up, adding one proton at a time to get through all of the known elements.
But the table is also
periodic
. It reflects the fact that as protons are added, their corresponding electrons can only be added into defined energy levels. Most of chemistry deals with the outermost electrons, since those are the ones that interact with other atoms. And every time an energy level is filled, the next electron added becomes a single outer electron, in a higher energy level. That makes the element behave like the one above it in the periodic table, because all of the elements in a column have the same number of electrons in their outermost shell.
This periodicity allows us to predict the behavior of elements scientists have yet to discover. Before the elements scandium, gallium, technetium, and germanium were discovered, their properties were predicted because there were “holes” in the periodic table where an element should have been. Likewise, the element protactinium was predicted before it was discovered. The same goes for the element hafnium.
Some elements were named in ancient times, and we don't know the exact origins. Gold, silver, copper, lead, tin, iron, zinc, and carbon were all known to the ancients, and their names come from attributes such as their color, where they were originally found, or what their uses were. The word
carbon
comes from the Latin word for “coal” and
gold
comes from the Latin word for “yellow.”
Other elements were discovered by chemists, who gave them names that we have some history for.
Hydrogen
means “water maker” because when it burns, we get water. It was discovered by Henry Cavendish but named by Antoine Lavoisier in 1783. Helium was first discovered by its spectral lines in the sun and was named after the Greek word for the sun.
Potassium was discovered in 1807 by Humphrey Davy and named after the material it was extracted fromâpotassium
hydroxide, or potash, which was literally pot ash, the ashes that remain in a pot after burning vegetable matter.
Other elements were named after countries or continents: francium, polonium, europium, gallium, germanium, and americium. Some were named after people: rutherfordium, seaborgium, bohrium, meitnerium, roentgenium, copernicium, curium, fermium, einsteinium, mendelevium, nobelium, and lawrencium. Uranium, neptunium, and plutonium were named for planets. Palladium was named after an asteroid, which was named for a Greek goddess. Other gods gave thorium, selenium, and mercury their names, although the last may have been named for a planet that was named for a god.
Some elements are named for the towns, universities, or laboratories where they were discovered: berkelium, dubnium, hassium, darmstadtium, hafnium, terbium, ytterbium, erbium, yttrium, and californium.
By premixing the gas with air. When gas is allowed to escape into the air through a tube and then set alight, it burns with a yellow flame, called a
luminous flame.
It is luminous and yellow because it produces carbon soot that is heated until it glows yellow.