Why Is Milk White? (8 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Tanning is a complex operation, involving steps to remove fats and hair from the skins, adjusting the acidity of the skins, and then allowing the skins to soak in a tanning solution. The tanning solution can use
tannins
, a kind of acid found in oak bark and other plant tissue, or it can use chromium compounds, which tan the hide faster and make a leather that is more resistant to shrinkage.
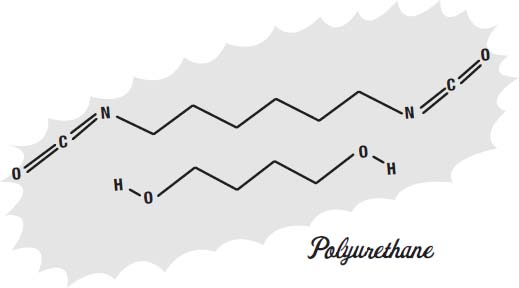
The soles of shoes can be made of leather but are increasingly made from synthetic materials that offer better traction, durability, and water resistance. Some of the materials used are ethylene vinyl acetate, rubber, thermoplastic rubber, rubber foam, and polyurethane.
Thermoplastic rubber is actually two compounds mixed together. One is styrene, the plastic that model airplanes are made out of. The other is a synthetic rubber called butadiene. The word
thermoplastic
means that the material can be melted and poured into molds, making it easy to form shoe soles with traction patterns in them. It also means they can be recycled.
Ethylene vinyl acetate is also a mix of two
polymers.
It is what hot glue sticks are made of. It is also what many foam rubber items are made from.
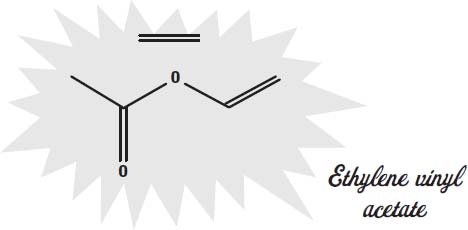
The molecules shown on this page are the
monomers
of the plastic. These monomers are the molecules that link together into long chains to form the polymers that give the plastic its solid form, its rubbery texture, and other qualities.
Perfumes in deodorant mask odors. Moisturizers make the skin feel soft and make the product glide onto the skin. Oils are added to make the deodorant more transparent so that it hides better on skin and clothing. Silica is added to absorb skin oils that accumulate from sweat.
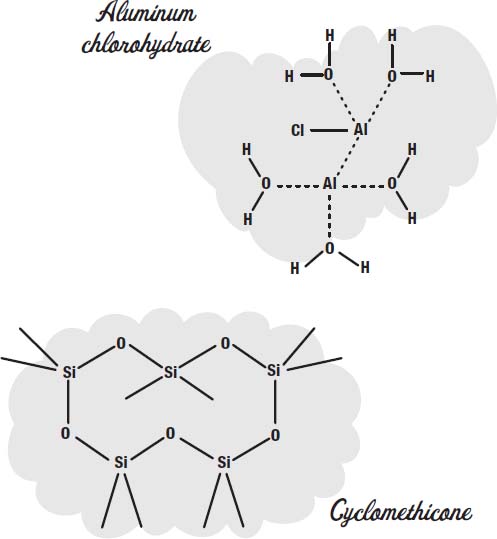
To control perspiration, salts such as aluminum chlorohydrate or aluminum zirconium tetrachlorohydrex GLY are used. These dissolve in the sweat and make a gel that coats the sweat glands.
Aerosol deodorants contain propellants, which are gases under atmospheric pressure but may be liquids in the deodorant can. Butane and isobutane are liquids under pressure or at temperatures you can reach in your freezer. Propane is a gas in the can or in the freezer and is added to the butane to get a higher pressure.
The mix of gases is tailored to the exact pressure needed to propel the other ingredients out of the can without spraying too hard.
In addition to the propellant, a carrier fluid is used to carry the ingredients out in the spray. Cyclomethicone is one common carrier fluid used in aerosol deoderants.
Roll-on deodorants usually have water and alcohol to dissolve the ingredients. The alcohol gives a cooling effect as it dries.
In solid antiperspirants, the ingredients are blended into a solid carrier, which might be a hydrogenated oil or a fatty alcohol like stearyl alcohol.
Mica. Lipstick is a soft crayon made of waxes like beeswax, carnauba wax, or candelilla wax, vegetable oil, and pigments. Lanolin is sometimes added, and talc is sometimes used also. Vitamin E is added to protect the vegetable oils from going rancid. But to make the lips shine, tiny flakes of the mineral mica are added. Mica gets its name from the Latin word for “shine.” The shiny gold flakes you see in sand at the beach are made of mica.
Matte lipsticks are mostly wax and pigment. Sheer lipsticks have less pigment. Glossy lipsticks have (in addition to mica) more oil and sometimes use a silicone-based oil to be longer lasting. Frosted lipsticks have a pearlizing compound made of bismuth oxychloride.
Other glossy compounds used are diisostearyl malate and triisostearyl citrate. These are
esters
, which are compounds made from alcohols and acids, in this case the fat called stearic acid. They give a wet look to the lip gloss.
Most lipstick pigments are shades of red. The most popular red pigment used in lipsticks is carmine, a dye made from the shell of a tiny scale insect that lives on cactus in the US Southwest.
The dye itself is carminic acid, which is used in many foods and cosmetics. It is sometimes called cochineal extract. Only the female insect has the dyeâa natural defense to keep other insects from eating the little critters. They sit on the cactus with their little straws sipping cactus juice, looking like little scales (which is why they are called scale insects). They can't run away, so they make carminic acid, which other bugs don't like.
Cochineal extract is also used in fruit juices like ruby red grapefruit juice, strawberry orange juice, pomegranate cherry juice, and many others. Like any other natural color derived from living
things, cochineal extract usually contains some proteins from the original source, and some people have allergic reactions to the proteins. For this reason, and because some people don't like the idea of eating bugs, some manufacturers are moving to synthetic dyes like FD&C Red #40. But that dye has a slightly more orange color.
Other red pigments used in lipsticks are made from iron compounds. In other words, the red comes from rust. Both red iron oxides and black iron oxides can be used. White pigment for pink lipstick comes from titanium dioxide or zinc oxide. Synthetic dyes such as D&C Red #6 are also common.
The size of the pigment particles has an effect on how the light hits the eyeâand thus, how it looks. Mica particles coated with colored metal oxides are particularly affected. Particles smaller than 25 microns produce a silky effect. Larger sizes up to 50 microns appear pearlescent. Larger sizes than that appear brighter and sparkly. Some lipsticks have metallic silver, gold, or copper colors made from mica particles coated with pigments.
Drano is made of lye (sodium hydroxide), mixed with little bits of aluminum metal. When lye crystals mix with water, they react strongly and produce a lot of heat. The hot water softens lumps of grease in the drain. The sodium hydroxide then reacts with the softened grease, turning it into soap. The bits of aluminum metal also react with the sodium hydroxide, releasing more heat but also releasing a lot of hydrogen gas, which makes bubbles that further loosen the fat and grease in the clog.
The hot water dissolves the soap. The bubbles of hydrogen break up the lumps of grease and make them float up away from the clog, so more hot water and lye can react with the grease and fat.
If there is hair in a clog, the lye also dissolves that, and the hydrogen bubbles loosen the strands from one another, so that the clog catches more water and is carried down the drain.
So there are many chemical reactions going on at once. Heating the water, making soap, making bubbles, and dissolving proteins. Add to this the force of new water pushing on what remains of the clog, and lye-based drain cleaners do a pretty good job.
Lye can cause burns to skin and eyes, so caution should be used with any product that contains sodium hydroxide.
Hairsprays use propellants. A
propellant
is a gas or a liquid under pressure. When the pressure is released, the liquid boils or the gas expands, carrying with it any other ingredients in the can. In the case of hairspray, those ingredients are the polymers (basically glue) that hold the hair in place.
But propellants are used to get other things out of spray cans as well. And the contents of the can dictate which propellant is used.
For example, hairspray uses propane, butane, and isobutane as propellants, because the polymers in the spray dissolve easily in butane, which is a liquid under the kind of pressures in the can. Propane also dissolves in butane, so less pressure is needed to contain a lot of propane in the can. The mix of the three propellants is adjusted so that the spray comes out at just the right pressure. Too much propane, and the spray comes out too hard. Too little, and there is not enough force to carry the ingredients to your hair.
Some hairsprays use dimethyl ether or methyl ethyl ether as propellants. These are similar in function to butane, and like butane, are flammable.
The propellant in whipped cream is nitrous oxide (laughing gas). This gas dissolves easily in fats and is also used for cooking sprays. Using a gas that dissolves in the other ingredients in the can allows a lot of gas to be put into the can without using a lot of pressure. When you release the pressure on a can of whipped cream, the cream expands to four times its size in the can. But if air was used, the cream would only whip to half that volume. Air would also allow the cream to go rancid, since it has oxygen in it. Carbon
dioxide would dissolve in the water in the cream, but then it would make carbonic acid, which would curdle the proteins in the cream.
Other propellants are pure nitrogen (it doesn't react with ingredients, but it doesn't dissolve well, so higher pressures are needed), and hydrofluorocarbons (they liquefy easily, they are nonflammable, they don't create smog, and the new ones are safe for the ozone layer).
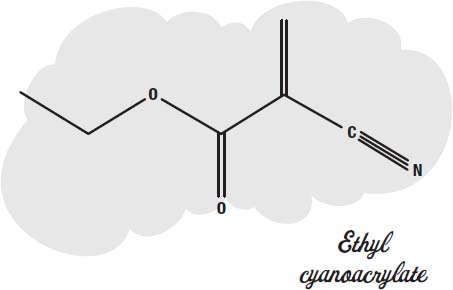
Superglue is made from a small molecule called
ethyl cyanoacrylate.