Why Is Milk White? (21 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

The presence of unburned carbon soot is an indication that the gas is not burning completely and some of the energy available in the gas is wasted. The gas is not mixing well with the air before it burns.
If some air is mixed with the gas before it reaches the flame, then more of the gas will be able to burn, and the result will be a hotter flame without any soot. Hotter flames burn blue instead of yellow and are not as bright, because there is no soot to heat to incandescence.
To mix air with the gas before it reaches the flame, a gas stove has a small opening for the gas, to make it jet out quickly into the air. It also has holes in the tube so the jet of unburned gas can draw
in air through what is called the Venturi effect. The air and the gas mix thoroughly in the tube as the mixture travels to the openings in the burner. The openings are small holes, so that the flame does not travel back down into the tube. You can see the flames are blue and have little or no soot, and they start a little bit away from the holes in the burner.
Other gas heating devices work the same way. If you have a gas heater in your house, it premixes the air before igniting it. A gas clothes dryer also does this. In a chemist's laboratory, the Bunsen burner also has a small gas jet that sucks up air from holes in the tube and mixes the air and gas before burning it at the far end of the tube.
To get the hottest flames, a large amount of air is used, so that all of the gas burns. To allow this to happen without igniting the gas and air too early and in the wrong place, a screen of metal can be used to separate the flame from the gas and air mixture. The flame cannot get past the screen, since the metal draws off too much of the heat. A burner with this design is called a Meker burner, and this concept is also used in gas stoves for the kitchen, where the tiny exit holes act as the screen.
and â¦
Why is oil so oily?
Oil lubricates things. A
lubricant
is a film of liquid that prevents parts from touching, so they slide easily past one another.
When you get a lubricant on your fingers, one finger can't feel the otherâthey only feel the lubricant. They slide past one another, and we say they feel oily, since most oils make good lubricants.
Lubricants generally work because their molecules bind more tightly to the parts that would otherwise rub together than they do to one another. The oil added to machines sticks to the metal
parts, so it stays on the metal as a coating. But the coatings themselves do not stick to one another and instead just glide by.
Oil on your fingers works the same way to make your fingers slip past one another and thus feel slippery. Soap is also a good lubricant for fingers because one end binds to the skin and the other end is an oil, which does not bind to the oil ends of other soap molecules.
Many lotions contain oils. This is to prevent moisture loss from the skin, which a coating of oil will do. What people don't like is a greasy feeling. Grease is a thick oil or fat that is only a lubricant when under pressure. It is thicker and does not allow the skin surfaces to easily slide past one another.
Unsaturated oils can oxidize, which can make them form a plastic film that feels sticky and unclean. Lotions fight this in two ways: by adding ingredients that prevent oxidation, like vitamin E (tocopheryl acetate) and vitamin C (ascorbic acid, or ascorbyl palmitate), or by using saturated oils like mineral oil, which stays liquid and does not oxidize.
Most moisturizing lotions actually don't add much moisture to the skin. Instead, they use oils and fats to prevent moisture loss. After all, it is easy to moisturize your skin in a shower or a bath. The problem is keeping it moist after you leave the water.
Balloons are made of latex rubber. Latex rubber is a polymerâ that is, a material made from long molecules that tangle up like spaghetti. And just like spaghetti, there are spaces in between the molecules that can let smaller molecules through.
The real question about balloons is why it takes so long for the air to leak out. In 1831, a man named Thomas Graham figured it out. It turns out that all gas molecules have the same average energy if they are at the same temperature. The energy of a gas molecule is a combination of how heavy it is and how fast it is moving. That means that if there are two molecules at the same
temperature, but one is lighter than the other, the lighter one must be moving faster.
Fast-moving molecules leak faster than slow-moving ones.
The lightest gas molecule is hydrogen. It has an atomic weight of 2. The second lightest is helium, which has an atomic weight of 4. To find out how much faster hydrogen leaks from a balloon than helium, divide the square root of the heavier weight by the square root of the lighter one. So hydrogen leaks 1.414 times as fast as helium.
An oxygen molecule has an atomic weight of 32. It stays in the balloon four times as long as hydrogen. Air is mostly nitrogen molecules, with an atomic weight of 28. Nitrogen stays in the balloon 3.74 times as long as hydrogen.
One would expect butane, a gas whose molecules have four carbons and ten hydrogens, weighing 58, to stay in the balloon over five times longer than hydrogen, and 1.414 times longer than air (which has an average weight of about 29). But butane leaks out of the balloon surprisingly fast. I suspect that the butane is reacting with the latex rubber, either making it stretch out more, so it is thinner, or actually dissolving the rubber a little bit, so the long molecules are separated by butane molecules, which can then leak out quickly.
There are several ways to interpret the question, and so there are several answers. That makes it more fun.
It is not good to get acid in your eye. It hurts. The acid changes the shape of the proteins in your eye and skin, so those proteins don't work the way they normally do. Your body responds to this by sending pain signals to your brain, so you stop putting acid in your eye.
In your stomach, acid is produced by your body to help you digest your food and to kill microorganisms that might make you sick. It changes the protein called pepsin into the right shape to help break down the proteins in your food. Stomach acid also helps break the food down into smaller pieces, so it can be absorbed in your intestines.
BUTANE BALLOON
Adult
supervision
required
Materials
Butane lighter refill can (available at drugstores or hardware stores)
Freezer
Balloons (preferably large, 12-inch diameter) Oven mitts
Kitchen scale (optional)
I love showing people how to do fascinating new things with ordinary objects and materials they might have around the house. This project plays with butane and has some very interesting results without ever even lighting it on fire. Butane is flammable, though, so throughout this experiment, make sure to
keep it away from open flames.
You will need some time for preparation. An hour ought to do, but most of it will be just waiting. You also need a butane lighter refill can and some balloons. The large 12-inch-diameter balloons will work best.
First, put the can of butane into the freezer. Make sure the freezer is set to its coldest setting (this will also make your frozen food last longer). You want the butane to be as cold as the freezer compartment can make it.
Butane has a very interesting property. It is a liquid at -0.5° Celsius (31.1° Fahrenheit). Above this temperature, the liquid butane will boil, becoming a gas.
You may have seen transparent butane lighters, where you can easily see the level of butane liquid in them. Butane is a liquid at room temperature when it is under pressure. It does not take a lot of pressure to keep butane liquid. At 2.6 atmospheres
of pressure, it will remain liquid up to 100° Fahrenheit (39° Celsius). This is why it can be stored as a liquid in little clear plastic lighters.
On a cold winter's day, when the temperature is a few degrees or more below freezing, a can of butane (or a butane lighter) won't work very well, since the contents will remain a liquid and won't have enough pressure to leave the container. Some camping stoves run on a mixture of butane and propane for this reason, since the propane will still be a gas in cold weather.
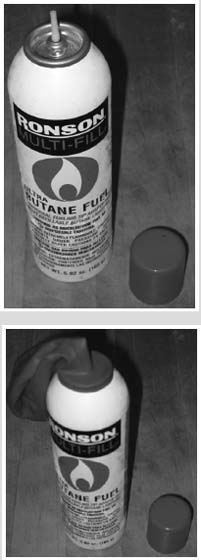
If the freezer has been set low enough, it will only take an hour or less for the butane can to get so cold that the butane will be a liquid.
Remove the butane can from the freezer using a towel or some oven mitts so your hands don't warm up the can. Stretch a balloon over the top of the can.
Turn the can upside down, pinch the plastic nozzle through the neck of the balloon, and push it toward the can. Liquid butane will pour into the balloon, along with some gaseous butane that has warmed up a bit. As the butane hits the balloon, some of it will boil into butane gas. The balloon will start to inflate a little.

Pinch the neck of the balloon and remove it from the can, and tie it closed.
The first thing to notice is that frost forms on the outside of the balloon where the liquid butane has made the rubber very cold.
Hold the balloon in your hand. The cold liquid butane will boil from the warmth of your hand. You can feel it boiling, even though it is below freezing in temperature. It makes a hissing sound, and the bubbles vibrate the balloon.

The balloon continues to get bigger. At some point, all of the liquid butane has turned into butane gas. As the gas warms to room temperature, the balloon will get a little bit bigger, but not as quickly as it did when the liquid was boiling.
The next thing to notice is that the balloon feels heavy. This was not surprising when it had a puddle of liquid in it and it was small, but now it is a big balloon full of gas. Yet the weight has not changed perceptibly.

If you have a kitchen scale, you can investigate further. An empty balloon weighs about 3 grams.
A balloon full of air still weighs about 3 grams. But a balloon full of butane gas weighs 12 grams.
Butane is denser than air, so any volume of butane will weigh more than that volume of air. And since air is neutrally buoyant in the air around us, it does not register on the scale. But the butane sinks in air and presses down on the scale.
The third fascinating thing to do with the butane balloon is to hold it up to your ear. The dense gas in the roughly spherical balloon acts as a lens for sound waves. Turn your head (with the balloon still against your ear) until some source of sound, like a radio or a television or someone talking, has to go through the balloon to get to your ear. When the balloon is directly between your ear and the sound, the volume suddenly gets louder. The balloon lens is acting like a telescope for sound.