Why Is Milk White? (30 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Next, take some string or some wooden skewers and dip them into the liquid. You can let the liquid dry on the string to form seed crystals, or you can roll them in granulated sugar and let them sit to dry for a bit. Drying allows the crystals to form in the crevices of the string or wood, so they are well anchored.
Fill some jars with the remaining liquid.
Now hang the string or skewers in the jars, being careful to keep them above the bottom of the jar. Crystals will form on the strings or skewers, but they will also form at the bottom of the jar and at the surface of the liquid. If the string or skewer touches the bottom, the rock candy crystals on the string will join those at the bottom, and you will get a big mass that you can't remove.
I like to put the jars into saucers of water so they don't attract ants. I also cover them with paper so that flies aren't attracted to the syrup. After a week, as the water slowly evaporates, the sugar will be forced out of solution and will crystallize on surfaces in the jar, such as the string or wood, that were seeded with small sugar crystals. Crystals grow first where there are already seed crystals.
Grow your crystals for 7 to 14 days and remove them when they are the size you like. As crystals form at the surface of the syrup, you can knock them down into the jar or remove them.
The biggest crystals will grow in a cool solution that is placed in a location free of vibrations from running feet or slamming doors.
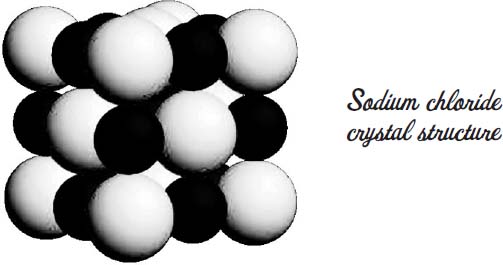
Because salt is made of tiny crystals in the shape of cubes.
Salt is made of sodium and chlorine. Chlorine atoms are a little bigger than sodium atoms. Chlorine is 181 picometers in radius, and sodium is only 102 picometers. They have opposite charges, so they pack as closely as they can, given their different sizes. That turns out to be the shape of a cube.
The salt in your salt shaker may have been ground up from larger crystals, so many of the crystals may be broken, especially if you have a salt grinder instead of a salt shaker. But many salt companies crystallize their salt to purify it and arrange the conditions of the crystallization so that perfectly sized little cubes are formed.
You can use a magnifying glass or a microscope to see the tiny cube shapes. Or you can dissolve as much salt as you can in boiling water and let some of it slowly evaporate in a glass dish. If the evaporation rate is slow (because you let the solution cool in the
refrigerator) you will get larger cubes that you can see without a magnifying glass.
Remember the talk about why ice and snow melt (
page 169
)? The same processes (of equilibrium at a given temperature) occur in most materials. When the temperature rises above the melting point, molecules leave the solid and enter the liquid at a faster rate than the molecules in the liquid crystallize onto the solid.
The fat in cocoa beans (called cocoa butter) melts in a narrow temperature range, between 93° and 100° Fahrenheit (34° to 38° Celsius). Normal human body temperature is in this range. So if the temperature is hot enough to melt chocolate, your body has a hard time staying cool, and you move into the shade. So chocolate is a solid because you live in a comfortable place.
Part of what we like in chocolate is that it is a solid in the wrapper on the table but becomes a soft paste or liquid in the mouth.
Other fats have different melting points. Tristearin is a hard, waxy fat that melts at 154° Fahrenheit (68° Celsius). Triolein, which has three kinks in the molecule, so it can't form compact crystals as easily as tristearin, melts at 41° Fahrenheit (5° Celsius).
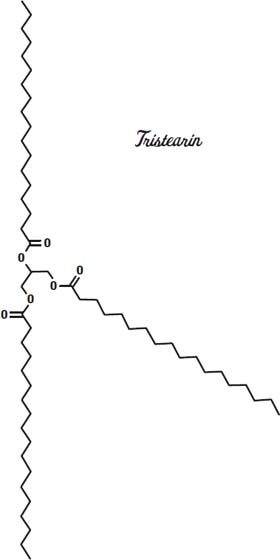
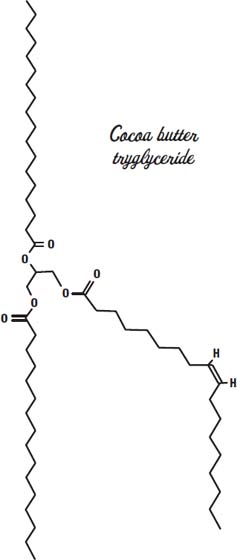
Cocoa butter has a mix of fats but is made mostly from the fatty acids stearic acid, palmitic acid, and oleic acid, sometimes on the same glycerin molecule, as shown on the previous page.
This arrangement gives it a melting point in between that of the solid tristearin and the triolein, which is liquid at room temperature. So our chocolate bar stays solid as long as we don't leave it out in the sun.
Well, some types are chunky. But disregarding the larger bits of peanut in your peanut butter, the creamy texture of it is due to the fact that peanuts are mostly made of peanut oil.
The peanut butter you get in the store is made of very finely ground peanuts, so your tongue cannot feel the individual particles. They are surrounded by peanut oil, which is a liquid. The fine peanut flour absorbs the oil, and the result is a thick paste.
Of course peanut butter does not resemble cream in either color or consistency. The producers of peanut butter call it creamy because that sounds better than pasty.
Peanut butter contains very little moisture. In your mouth, it takes a while to eat peanut butter because you have to moisten it before you can swallow.
Yes. But of course, there are chemicals in natural flavors too ⦠usually the same ones that are in artificial flavors.
A common artificial flavor is vanillin. Natural vanilla flavoring is a mixture of hundreds of different flavor molecules, but the most prevalent molecule in it is vanillin. Since vanillin can be synthesized much more cheaply than it can be extracted from vanilla beans, the synthetic form is much more widely available.
A more potent form of the molecule is ethyl vanillin. Both of these synthetic molecules are used to add flavor to foods such as chocolate, where the subtle differences between the natural and the synthetic molecules would be lost among all of the other strong flavors.
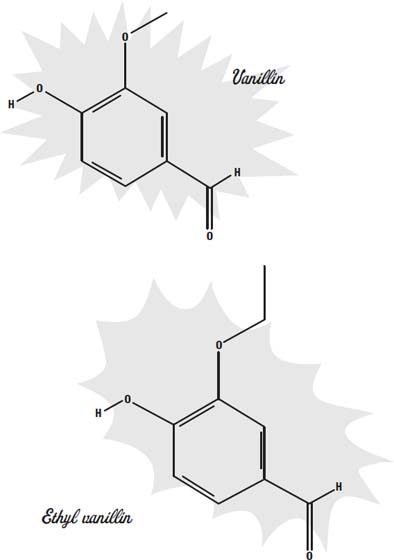
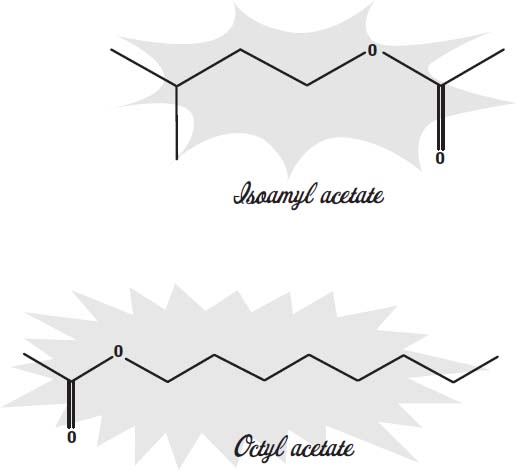
Many other artificial flavors have been synthesized. Most of them are esters, a compound made from an alcohol and an organic acid. As with vanillin, these molecules are the same ones found as the principal flavorings in natural flavors. For example, isoamyl acetate is the main molecule that gives bananas their flavor and aroma.
Octyl acetate is the main flavor component in oranges.
Carbonic acid.
Actually, when carbon dioxide is dissolved in water, only a small portion of it reacts with the water to form carbonic acid.
Under pressure, more and more of the carbon dioxide reacts, creating more of the acid.
In a soda can or bottle, the pressure is two and a half times as high as normal atmospheric pressure, and the acidity starts to approach that of orange juice. This makes carbonated water taste a little tartâsour, like orange juice. When the bottle is opened, the pressure drops to normal atmospheric pressure, and the carbonic acid slowly turns back into water and carbon dioxide. This releases the bubbles you see in your drink.
The bubbles in your drink form around
nucleation sites.
These are places where the solution has a large contact area with a surface, generally sharp points, edges, scratches, or crevices. If you have a large number of sharp points and crevices, the nucleation proceeds rapidly.
Champagne glasses sometimes have small scratches placed in the bottom to make a nice looking stream of bubbles flow up the glass. The scratches act as nucleation sites. Another now-famous example of nucleation is dropping Mentos candy into a carbonated beverage and watching the rapid, almost violent release of carbon dioxide from the millions of nucleation sites the candy offers.
DANCING RAISINS
Materials
| Dried blueberries | Raisins |
| Dried cherries | Flavored sparkling water |
Sometimes an extremely simple demonstration can stimulate quite a bit of thought.

I had my assistant drop some dried blueberries, dried cherries, and raisins into a glass of flavored sparkling water. They all quickly sank to the bottom, surrounded by a cloud of bubbles. After a short while, the raisins began to float up. When they reached the top, they sank back down to the bottom again, to start the process all over. They looked like wrinkled little submarines, bobbing up and down.
But the dried cherries and dried blueberries just sat there at the bottom.
The questions began very soon. What makes them go up? What makes them fall down again? Why do only the raisins seem to be active?

The carbonic acid in the soda water is slowly converting to water and carbon dioxide gas, because it is no longer under high pressure in the can. The easiest way for the gas to leave the water is at the water's surface. Making a new bubble from scratch takes more energy than simply enlarging an existing bubble.
At the top of the glass is a large surface open to the air. Most of the carbon dioxide gas escapes at that large surface. But as the dried fruit was dropped into the soda, bubbles of air were trapped in the wrinkled surface of the fruit. These bubbles of air act as extra surfaces, and the carbon dioxide gas escapes into them, making the bubbles bigger. When the bubbles are big enough, they rise to the surface. If they are stuck to a raisin, they can lift the raisin to the surface with them.
Once the raisin is at the surface, the bubbles continue to grow, and they either detach from the raisin or they pop (since they are now at the surface). In either case, the raisin no longer has enough gas to keep it afloat, and it falls back down. Usually the bubble that popped or detached left a tiny remnant of itself stuck to the raisin, and that tiny bubble starts to grow again.
But why did the dried cherries stay at the bottom?
The cherries are much like the raisins, but they are larger. This makes something interesting happen. Imagine a box just big enough to hold a marble. It has one marble in it, and it has six sides that can collect bubbles like the raisin. Now imagine that we double the width, height, and depth of the box, so that eight marbles can fit into it. The box now has eight times the weight but only four times the surface area to collect bubbles. Now there aren't enough bubbles to lift the weight. The dried cherries are just too big for the bubbles on the surface to lift.
But the small dried blueberries also stayed at the bottom. Why is that?
We looked closely at the blueberries. The bubbles there seemed to leave the surface of the blueberry before they got big enough to float it. It looked as though the surface of the berry was not as sticky to the bubbles as the surfaces of the other fruits. The blueberries also seemed to be a little denser than the other fruits, so there was more weight for each bubble to lift. Something about the surface of the blueberries seemed to be the most important thing, though. They just didn't have as many small trapped bubbles as the other fruits. It was like they were smoother or covered in a soap-like substance that allowed the water to wet them better.
All of this from a simple glass of soda and some dried fruit. Who would have thought?