Why Is Milk White? (13 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Since we can now get vitamin D from milk and supplements and we have effective sunscreens (and clothing and hats), there is little reason to risk cancer by excessive sun exposure.
and â¦
Why is it when you put hydrogen peroxide in your mouth it bubbles?
Hydrogen peroxide is H
2
O
2
. You can see that this is water (H
2
O) with an extra oxygen atom attached. Catalysts in your blood called
peroxidases
and
catalases
break it into water and oxygen.
Catalase is an enzyme (a protein the body makes that speeds up chemical reactions). It is very effective at speeding up the natural breakdown of hydrogen peroxide into oxygen and water. In each second, a molecule of catalase can break down 40 million molecules of hydrogen peroxide.
Your cells produce hydrogen peroxide as an undesirable side effect of breathing oxygen. Hydrogen peroxide is dangerous to cells, so they produce catalase to quickly break it down and escape damage. When you have damaged your skin, the cells that produce catalase are exposed. When you add hydrogen peroxide, the catalase in the cells breaks it down, and bubbles of oxygen form.
Hydrogen peroxide is used to damage germs that might find their way into damaged skin. If you use lots of hydrogen peroxide, the catalase can't keep up, and the bacteria get damaged. Some skin cells also get damaged, which is why the peroxide stings a
little, and peroxide is no longer recommended as a disinfectant for wounds now that better alternatives are available.
Other organisms also breathe oxygen, and so they need their own catalase enzymes. You can see them in action if you add some dried yeast to a cup of hydrogen peroxide. The peroxide starts to bubble vigorously as the oxygen is produced.
The active ingredients in cough syrup are drugs derived from opium. Coughing is caused by the brain, and these drugs act on the brain to suppress the urge to cough.
Opiates are a type of bitter-tasting chemicals called alkaloids. These are molecules that contain basic (alkaline) nitrogen atoms. Some alkaloids you may know include caffeine, nicotine, codeine, and quinine. Others are cocaine, morphine, heroin, ephedrine, atropine, dextromethorphan, pseudoephedrine, and strychnine.
Because the ingredients are bitter tasting, cough syrups have strong flavors to mask the bitterness. But that is not the only reason cough syrups are unpleasant to use. Because the ingredients in cough syrup include things like dextromethorphan and codeine, which are used by some people as recreational drugs, the pill forms have been taken off the market. Instead, the syrup forms are available, since they are much harder to use for purposes other than preventing coughs.
In other words, cough syrups taste bad on purpose, so people don't misuse them.
Besides preventing people from misusing certain drugs, liquid drug delivery systems are sometimes preferred because they are faster acting than pills. An extreme example is nasal spray, which acts very quickly because it can be delivered right into the nose and
then to the brain, without first having to be digested in the stomach and intestines to get into the bloodstream.
Pills make bad tasting medicines easy to swallow, since only a little of the medicine actually touches the tongue before the pill is swallowed. But pills and capsules can also be designed to pass through the acidic stomach without being harmed, so they can dissolve in the alkaline intestines and be absorbed into the bloodstream. Stomach acids can break down some medicines and prevent them from doing their job.
Pills and capsules can also be designed to dissolve slowly, so that they release small amounts of the medicine over a long period of time. This allows the patient to take one pill and get relief from symptoms for a whole day.
Some things we take in pill form are liquids. An example is vitamin A. It can be absorbed into powder to make a pill, but it can also be enclosed in a soft gelatin capsule that dissolves in the stomach to release the oily liquid vitamin.
There are several things going on that make the Band-Aid stick. This is true of almost any glue.
Mechanical adhesion is like Velcro. Two rough surfaces have many little places that catch onto one another, like hooks catch loops.
Electrical adhesion is where one part of a molecule is positively charged and is attracted to the negative charges of another molecule. It is a form of chemical adhesion, in which chemical bonds are formed between the glue and the surfaces that are being glued.
Atmospheric adhesion is like suction cups. Air pressure holds suction cups to smooth surfaces like glass.
To be a good glue, something must stick well to the surface it is applied to (this is called
adhesion).
But it must also stick well to itself (this is called
cohesion).
For example, water has good adhesion. You can wet two pieces of paper and glue them together with
just water. But water does not have good cohesion, and you can pull the pieces of paper apart and water will remain on both of them, because the water did not stick well to itself.
In a Band-Aid, you want the glue to stick very well to the Band- Aid and pretty well to itself, but you don't want it sticking too well to the skin or it will hurt to pull it off, and there might be some glue left on the skin that you would have to wash off later.
So Band-Aids have a special glue that doesn't hold on to the skin as well as superglue would. But it holds on to the skin a little better than sticky tape used for paper would, so the Band-Aid stays on long enough to do its job.
Psychologists use the term
psychosis
instead of “crazy.” The brain works by using chemicals to send signals between nerve cells. As mentioned in
chapter 1
, these chemicals are called
neurotransmitters.
If a person has a lack of some neurotransmitters or an excess of one or more neurotransmitters, they may perceive things differently from other people.
One example of this is hallucinations, where they see or hear things that are not there. But other examples are more common, such as depression and bipolar disorders, where the person feels sad or irritable or has an elevated arousal or energy level.
Some drugs affect the same receptors in the brain that natural neurotransmitters affect. This is why they have effects on the brain and why we use them to make up for a lack of a neurotransmitter in people whose bodies aren't making enough. Other drugs block the receptors that neurotransmitters use, so the person experiences the same effects as if they had little or none of the neurotransmitter.
Drugs that can strongly affect how the brain reacts to neurotransmitters are called
psychotomimetic drugs.
This just means that they mimic the effects of psychosis. Often these drugs are
used (or abused) in small doses that do not trigger psychotic effects or behavior, but if taken in large doses the effects can be disabling.
An example is tetrahydrocannabinol, the active ingredient in marijuana. In massive doses taken intravenously, it affects the cannabinoid receptors in the brain to such an extent that symptoms of schizophrenia develop. Some opiates are also psychotomimetic, such as pentazocine and butophanol. Other alkaloids that can be psychotomimetic are scopolamine, atropine, diphenhydramine, phencyclidine, and dextromethorphan. Psychotomimetic drugs may cause symptoms of depression or euphoria and dreamlike states in which things are not clear and sharp.
Most of the contents of hairspray are propellants and carrier fluids. Some propellants and carriers, such as hydrofluorocarbons and silicones, are fairly harmless and merely reduce the amount of oxygen you breathe. This can make you dizzy, like holding your breath. These are called
asphyxiants.
Some common propellants like nitrous oxide, propane, and butane can actually affect how the brain works. Carrier fluids like alcohol and ether also affect the brain. The effects are caused by either stimulating or blocking receptors in the brain that are normally triggered by natural neurotransmitters.
The effects can be drowsiness or sleep, distortion of vision or hearing, emotional disturbances, or hallucinations. Other, more common effects are headache, nausea and vomiting, slurred speech, loss of control of the muscles and coordination, and wheezing. Prolonged or frequent breathing of aerosol propellants and carriers can result in rashes around the skin areas that are exposed to the chemical.
Death from asphyxiation (lack of oxygen) or from heart failure can result if large amounts of aerosol propellants are inhaled. But since aerosol propellants also get very cold as they expand, they
can freeze the delicate tissues in the lungs, nose, and throat. The lack of motor control can cause the person to inhale vomit and choke on it. Brain damage can also occur with prolonged inhalation. But breathing a little bit while using the hairspray is generally not a problem.
Hydrogen peroxide kills the germs that cause gum disease and helps remove particles of food and bacteria from between teeth. It also reacts with bad-smelling molecules that are produced by bacteria, so it freshens breath.
Brushing your teeth is good for removing the bacteria that have glued themselves to your tooth surfaces. It also reaches down into the space between the tooth and the gum to remove some of the food and bacteria that settle there. But it is not very good at getting deep below the gum line.
Flossing helps a lot. It scrapes off the biofilm that the bacteria use to glue themselves to the teeth, and the floss can slip way down between the teeth and the gums to reach where the toothbrush can't. Still, flossing can leave many of the germs behind.
Hydrogen peroxide reacts with the catalase enzymes that the germs produce, causing it to split into water and oxygen. The bubbles of oxygen then carry the bacteria and food particles up out of the gums where they can be spit out. There is so much hydrogen peroxide in a mouthful that it overwhelms the bacteria's ability to break it down, and the bacteria are killed.
Because the peroxide works by oxidizing the bacterial cell walls and contents, there is little that bacteria can do to develop resistance to it. This is unlike many other antibiotics, which work by targeting specific functions in the germs. The germs can evolve defenses against these more easily than they can against hydrogen peroxide.
That's not a good thing to do. Though the body produces hydrogen peroxide as a byproduct of cellular metabolism (breathing) and has catalase enzymes to break it down, swallowing a mouthful of peroxide will overwhelm the ability of the catalase to work. This will allow the remaining peroxide to damage cells in the same way it damages bacteria. The result is a sore throat and an upset stomach.
The peroxide will also continue to break down as the body produces more catalase enzyme, so you will get bubbles of oxygen gas forming in your stomach, which will cause you to burp.
Hydrogen peroxide oxidizes the eumelanin and pheomelanin pigments in hair. By itself, hydrogen peroxide will do some lightening, but for hair bleaching it is usually combined with ammonia. While any alkali will make the scales on the hair shaft open up to let the peroxide in, ammonia actually breaks down the little packages (called
melanosomes
) of melanin particles, allowing the bleach better access to them.
Melanins come in many forms, but most are similar to the molecule shown in the drawing on the next page. Like any dye or pigment, the ability to absorb a particular color comes from the alternating single and double bonds. Single bonds are shown in the picture as single lines, and double bonds are double lines.
When a molecule has single and double bonds right next to one another, the electrons that form the bonds usually spread out over both locations, so we actually think of the two bonds as being “one and a half” bonds. This is important, because in a dye these “delocalized” electrons resonate with the light, at a frequency that depends on how many bonds they can cover.
If there are lots of delocalized electrons in a molecule, the light they absorb will have a long wavelength, like red or infrared. If
there are only a few, the wavelength will be shorter, like blue or ultraviolet.
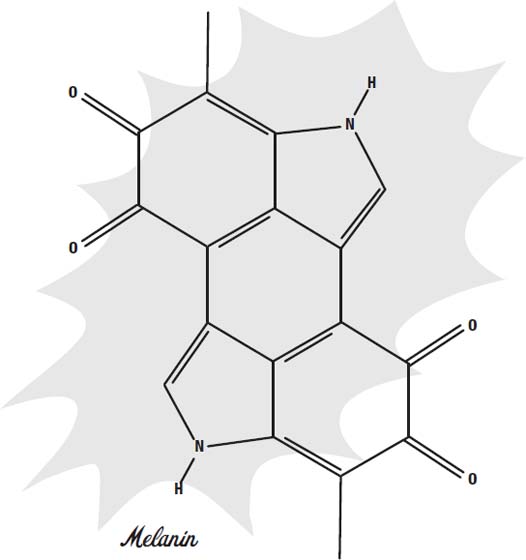
Reacting the molecule with hydrogen peroxide causes oxygen atoms to attach to the molecule. This usually turns one or more of the double bonds into single bonds. This means the molecule no longer absorbs light the same way and is either a different color or loses its color altogether.