Why Is Milk White? (25 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

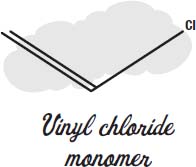
Plastic plumbing pipes are made from monomers of vinyl chloride, joined together to make polyvinyl chloride plastic.
The carbon atoms join together into long chains in which every second carbon has a hydrogen and a chlorine atom instead of two hydrogens.
Another common plastic monomer is vinyl acetate.
Polyvinyl acetate, made from this monomer, is familiar as white school glue. It forms a flexible, translucent plastic that can be heated to change its shape.
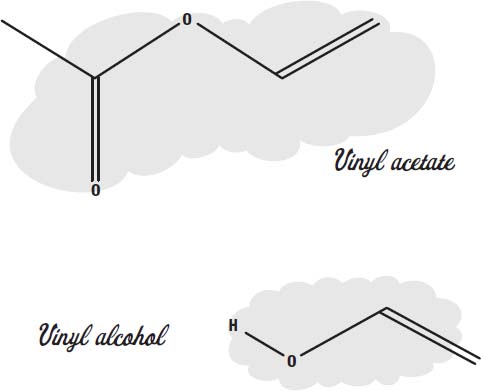
Between 100 and 5,000 vinyl acetate molecules join up to form the long chains in the polymer.
A related polymer is polyvinyl alcohol. It is made of monomers of vinyl alcohol. This plastic is often combined with other monomers to make what are called
copolymers.
Changing the type of copolymer or the proportion of the two monomers can change the nature and properties of the plastic produced.
FUN WITH BORON
Material
1 tablespoon borax (available at grocery stores in the laundry aisle)
1 cup hot tap water
Disposable plastic cup
1 tablespoon of Elmer's School Glue Gel (available at office supply stores)
3 tablespoons warm water
Standard white Elmer's Glue (optional)
Guar gum (optional, available at health food stores)
Talcum powder (optional)
Glow-in-the-dark or washable paints (optional)
Boron sits just to the left of carbon in the periodic table of the elements. Carbon, of course, is the molecule of life, able to make long strings of atoms called polymers. These make up things like DNA, protein, plastics, wood, and the fibers in our clothing. Boron is almost carbon. While it does not make long polymer strands by itself, it is quite useful for joining polymers together. When it does this, we call it
cross-linking.
Cross-linking makes a polymer firmer. Depending on how much crosslinking happens, the result can be an oozy slime, a bouncing ball, or a firm, hard plastic.
In this project, you are going to start with a boron-containing molecule called borax. You may have some in your laundry room.
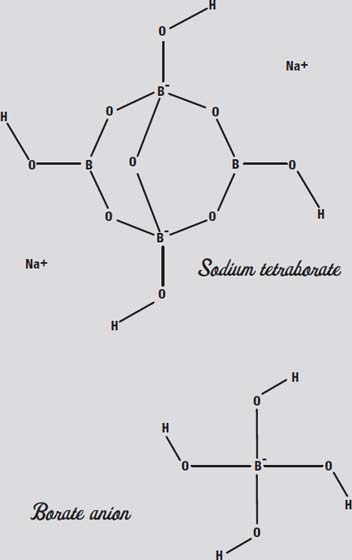
In water, borax dissolves to form the
borate anion,
a boron atom surrounded by OH (hydroxyl) groups.
Look at those four OH groups hanging on the boron. Those will be important in a minute.
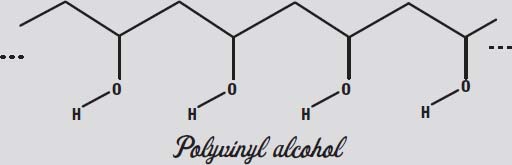
You have several choices of polymer to cross-link with our borate anion. The first one you will play with is called polyvinyl alcohol, or PVA for short. This is what we find in Elmer's School Glue Gel, the transparent blue glue you can find in office supply stores. You will play with the more common white glue, made from polyvinyl acetate (PVAc for short) a little later.
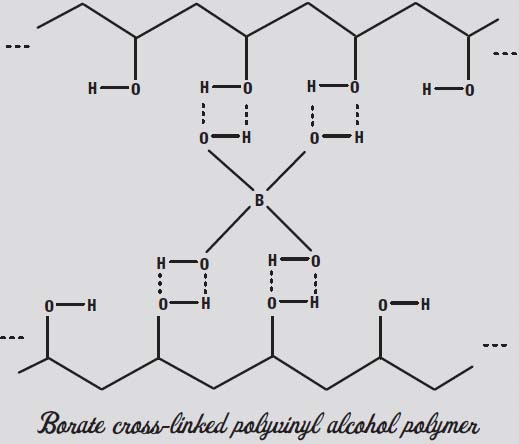
In the illustration below, notice that the polyvinyl alcohol (continued in the blue school glue gel) also has those OH groups dangling from every second carbon in the polymer chain. Those can interact with other OH groups to form what are called
hydrogen bonds.
In the OH group, the oxygen attracts the electrons more than the hydrogen does. This leaves the oxygen slightly negative and the hydrogen slightly positive. Two OH groups will thus be attracted to one another like magnets, with the positive end of one attracting the negative end of the other.
Now look at the illustration on the next page and see what happens when you add a borate anion to the PVA.
This form of cross-linking is easily broken, since hydrogen bonds are pretty weak as chemical bonds go. The result is not a strong plastic or a strong glue but a kind of slime that you might like to play with. But notice that two strands of the polymer
that used to be simply tangled up together like spaghetti are now joined by the borate, so you have something more like a net. A net is firmer than a tangle of strands.
To make slime, you will need a saturated solution of borax in water. That means you dissolve borax in hot water from the tap until no more will dissolve and then let it cool to room temperature. There will be some borax sediment at the bottom, and that tells you that it is a saturated solution. About a tablespoon of borax to a cup of water will do nicely. Feel free to use more borax; you can't screw it up by using too much.

In a separate cup (disposable plastic cups are nice for this step) pour in a tablespoon of polyvinyl alcohol (Elmer's School Glue Gel).
Using the same measuring spoon (so it gets cleaned a little bit) add three tablespoons of warm water, and stir. Rinse the spoon well after this.
The last step is to add a tablespoon of the borax solution and stir well.

The slime will thicken fairly quickly. Remove the thick mass and knead it in your hands for a while, stretching it and folding it. You can control how thick or runny it is by changing how much water there is in the mix.
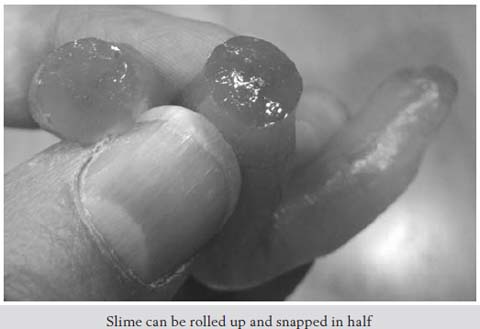
Left to itself, the slime will melt into a puddle. But you can pick it up, roll it into a ball or a rope, and snap it into pieces. Then let it rest, and it oozes back into a puddle.

You can make another polymer toy very similar
to Silly Putty by crosslinking polyvinyl acetate (standard white Elmer's Glue) with borax. Five parts glue, four parts water, and one part saturated borax solution.

Like the original, it can be rolled into a bouncing ball, stretched slowly, or broken with a sharp pull, and it can transfer ink from newspapers.

Other polymers can also be crosslinked using borax. Try guar gum for making a nice slime. Additives can be added, such as talcum powder or glow-in-the-dark or washable paints, to change the texture and color.