Why Is Milk White? (29 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

The diced apples in the oatmeal are made from apples and have calcium ascorbate added as an antioxidant to keep the color from changing. Calcium is an essential nutrient, and ascorbate is vitamin C. Many people think antioxidants are good for your health and that we should get more of them.
The cranberry raisin blend includes cranberries and raisinsâ and more sugar. It also has sunflower oil, and the dried fruit has been preserved with sulfur dioxide. Most dried fruit you eat is preserved this way. Some people are sensitive to sulfites, which are formed by the preservative, but most of the sulfur dioxide has dissipated by the time the product gets to the consumer.
The light cream part of the product has the most unfamiliar names. Milk and cream are in it, but so are sodium phosphate, diacetyl tartaric ester of monoglyceride, sodium stearoyl lactylate, sodium citrate, and carrageenan.
Sodium phosphate is one of the electrolytes added to sports drinks. It controls the acidity and stabilizes the proteins in the milk. Datem (diacetyl tartaric ester of monoglyceride) is an emulsifier made from vegetable oil and cream of tartar, which keeps the cream from separating from the milk. Sodium stearoyl lactylate is another emulsifying agent. Sodium citrate is a salt of citric acid (the stuff in orange juice), and it prevents milk from curdling by acting as an acid buffering agent, much like sodium phosphate. Carrageenan is a thickening agent (like a starch) that is made from seaweed.
With these ingredients the milk can be stored and transported without curds and lumps of fat forming. You will find these ingredients in single-serving coffee creamers for the same reason.
Your bones are made from calcium phosphate. So are your teeth. But your body uses calcium in other tissues, such as muscles, the heart, and the nerves.
When you are growing, your bones need to grow, and so you need calcium. If you get regular exercise, your bones feel the forces and respond by adding calcium phosphate to become stronger. If you do not get enough exercise, the bones weaken and can break more easily.
When your body needs calcium for the muscles and nerves, it can get it by dissolving bone tissue to get more calcium into the bloodstream. You will gradually lose calcium from your bones if you do not get more calcium in your diet. You also need vitamin D in order to process and absorb calcium from your food.
Most bone building occurs when you are young. Later in life, your body will use the calcium you stored in your bones when you were growing. In older people, and especially in women, the loss of calcium can make the bones weak and brittle. So it is important to get plenty of calcium, vitamin D, and exercise when you are young. Your body is banking calcium.
As you get older, less calcium is needed, since your bones are not growing. But getting adequate calcium to replace what is lost is still important, as is exercise and vitamin D.
Calcium is an element, so your body cannot make it but must get it from the environment. Besides vitamin D, other things that help absorb calcium from the diet are protein, magnesium, and phosphorus. Some foods, such as spinach, sugar, alcohol, and coffee, can prevent calcium absorption.
An adult woman has about two pounds of calcium in her body. An adult man has about three pounds. All but 1 percent of it is in the bones.
Calcium helps muscles contract, helps blood clot, controls blood pressure, and helps prevent gum disease. Lack of calcium can cause leg cramps, muscle spasms, and nerve sensitivity.
You eat food because your hormones tell you to. It is obvious that in order to grow, a body needs to accumulate the material out of which it is made. But adults who have stopped growing (at least vertically) still lose mass every day and must replace it.
But you also eat in order to get energy. You eat fats and carbohydrates mostly for the energy they provide. You can also burn proteins, but you mostly eat proteins to get the raw materials for making the proteins that our body is made from and that you use as catalysts (enzymes) to guide chemical reactions.
But the urge to eat, that need you feel when you are hungry, is due to hormones. The hormone
ghrelin
is produced in the brain but also in the pancreas and the lining of the stomach. It is produced at high levels before meals and decreases after meals. It makes you feel hungry.
Other molecules that make you feel hungry are
neuropeptide Y,
which is secreted by your intestines, and
anandamide.
Another hormone,
leptin,
is produced in fat cells. It makes you feel full. It affects your appetite and also how fast you burn energy. It acts by inhibiting the effects of neuropeptide Y and anandamide and increasing levels of a-MSH (alpha MSH), which is another appetite suppressant.
Other hormones and signaling molecules (such as
insulin
) are involved in regulating appetite and metabolism, and each of the hormones and signals are used in different places in the body to do different things. The science of how we regulate our metabolism is very complex and interrelated with other bodily functions in complicated ways.
Understanding the chemistry of how foods go bad allows us to design countermeasures to prevent spoilage. Some of those countermeasures involve knowing what kills bacteria and molds and what causes food to break down all by itself.
Living things need enzymes to do their job. Enzymes are proteins that use their particular shape to guide chemical reactions. If something changes that shape from its natural form, it is called
denaturing
the protein. A denatured enzyme usually does not work properly.
One way to denature enzymes is to heat them. When food is sealed in cans and then heated, all of the enzymes inside are denatured. That kills the bacteria and molds, since they need their enzymes to survive. And it also denatures the enzymes in the food, which would otherwise cause the food to break down into an unappetizing mush. It is enzymes that cause fruit to bruise and meat to spoil.
Bacteria and mold can also be prevented from growing by using chemicals. There are two classes of chemicals that prevent bacteria and molds from producing the energy they need to live and grow. These are the benzoates and the propionates.
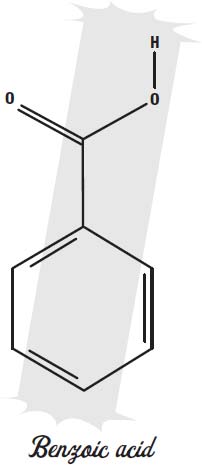
In acidic foods, benzoic acid or sodium benzoate can be used to keep bacteria and molds from spoiling the food. Benzoic acid and benzoates are found naturally in dried fruit, and they may be a natural antibiotic in those fruits. They prevent the microbes from fermenting sugar into alcohol.
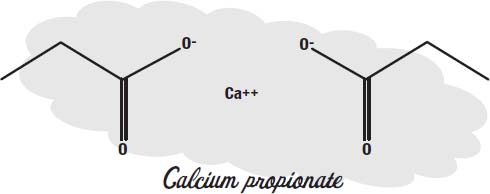
Benzoates only work in acidic foods. Calcium propionate is used in foods that are not acidic, such as baked goods.
Higher forms of life can metabolize propionates, but in bacteria and mold the molecule interferes with energy production.
There are many other molecules that harm bacteria and molds but not people and animals and are thus used to preserve food. In addition to those, molecules like salt and alcohol can be used in large quantities to kill germs but then be rinsed off before the food is consumed.
Plain water by itself does not usually boil over the edge of the pot. The reason for this is that the bubbles pop when they reach the surface.
If you are cooking pasta, the proteins from the pasta change the surface tension of the water. Proteins often have one side that is charged and attracts water and another side that is not, which sticks out into the air. In this way proteins act like the soap in a soap bubble. They stabilize the bubble, so it can live longer before popping.
If the bubbles don't pop, then new bubbles form underneath them and lift them up. Eventually they will spill over the edge of the pot and make a mess.
Adding a little oil changes this. The ends of the proteins that avoid water end up sticking into the oil instead of into the air. This makes the proteins unavailable to stabilize the bubbles. They are all locked up in the oil, making tiny oil droplets out of bigger ones, the same way soap does when you wash the dishes.
Adding oil to pasta coats the pasta with oil when you lift it out of the pot. This can interfere with the sauces, so they no longer coat the
noodles. You can pour out the surface water first, so the oil goes down the drain, or you can stop putting oil in the pasta and just watch the pot more carefully. If you turn down the heat when the water starts to boil over, you can prevent the mess without chemistry.
As long as the water is boiling at all, it will be at the temperature of boiling water. Boiling the water faster only makes the water evaporate faster. It does not speed up the cooking, since the temperature cannot get higher than the boiling point as long as there is still water in the pot.
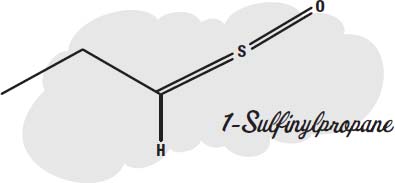
Because of 1-Sulfinylpropane.
This inconvenient little molecule is produced by enzymes in the onion when you cut it. Normally these enzymes are locked up inside the onion cells, but the knife breaks open the cells and lets the enzymes out, where they react with other molecules that are released, making 1-Sulfinylpropane.
Since 1-Sulfinylpropane is a small molecule, it can easily evaporate into the air. It gets into your nose and eyes, where the sensors in the eye that protect it from harmful substances get alerted. To flush out the bad molecules, the eyes and nose produce large amounts of liquid in an attempt to wash away the irritant.
Some onions produce less of this molecule than others. Onions grown in low-sulfur soils generally produce less, and there are sweet onions like the Vidalia that produce less of it.
You can prevent the tears in a number of ways. You can chill the onion in the refrigerator before cutting it. Enzymes work more slowly when they are cold. You can chop the onions under a fan, so the molecule never gets to your eyes and nose. You can chop the onion underwater (although just how practical does that sound?). You can wear your protective chemistry goggles, the ones that make a tight seal around your eyes. Or you can use a nice little onion-chopping gadget that chops them under a plastic container.
Rock candy is crystallized sugar. The crystals are usually grown slowly (over a week or two) to get large, attractive crystals. Growing crystals quickly makes a mass of small crystals instead of nice large crystals.
To get the crystals to grow slowly, you need to control the rate at which the sugar precipitates out of a sugar solution. If you boil the water out of the solution quickly, you get candy like taffy or nougat, where the crystals are so tiny you can't feel them on your tongue. If you disturb the candy by beating it, you also get billions of tiny crystals, as you do when you make fudge.
To make rock candy crystals, start with plain sugar and water. Two cups of sugar will dissolve in one cup of water if you boil the water. If you want to make more candy, you can double the recipe.
Boil the sugar and water together until all the sugar dissolves. Remove the pan from the heat. At this point you can add food coloring or flavors like vanilla, mint, cherry, lemon, grape, or strawberry. Let the liquid cool for at least 10 minutes.