Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (154 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md


14
C-
D
-xylose breath test has good specificity.
Hydrogen breath tests (glucose-H
2
, lactulose-H
2
)—not recommended because of limited sensitivity and specificity.
CELIAC DISEASE (GLUTEN-SENSITIVE ENTEROPATHY, NONTROPICAL SPRUE, IDIOPATHIC STEATORRHEA)
Definition
Celiac disease is an autoimmune multisystem disorder (principally manifested in the GI tract) in genetically susceptible persons that may be caused by a mucosal injury by a complex of gliadin (a protein from dietary gluten in wheat, rye, barley, or oats) with tissue transglutaminase (tTG), a cross-linking enzyme. Findings are caused by malabsorption and autoimmunity.
Laboratory Findings
Although there are no universally accepted tests for the diagnosis of celiac disease, specific serologic testing and small bowel biopsy are very sensitive and specific for making the diagnosis. All tests must be performed while patients are on a diet of food that contains gluten (Figure
5-5
).
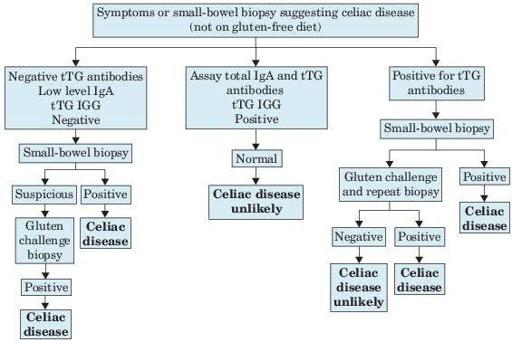
Figure 5–5
Symptoms or small bowel biopsy suggesting celiac disease. tTG, transglutaminase.
Histology
: Biopsy of jejunum is the diagnostic gold standard; shows characteristic although not specific mucosal lesions. Establishing the diagnosis is essential; patients should not be committed to lifelong gluten-free diet without first assessing intestinal mucosal histology. False-negative results may occur because of patchy distribution of pathology.
Stool findings
: Steatorrhea demonstrated by positive Sudan stain on ≥2 stool samples or quantitative determination of fat in 72-hour pooled stool sample.
Anti-human IgA tTG antibodies
: (by ELISA) has S/S = >90%/>95%. False-negative results may occur in patients with IgA deficiency (present in 2.5% of patients with celiac disease for whom corresponding IgG antibody tests may be useful). More reproducible than EMA test.
Anti-IgA deamidated gliadin IgG/IgA antibodies
: Deamidated gliadin antibody (DGA) recognizes an antigen related to dietary gluten and is responsible for initiating inflammation in celiac disease. Antigliadin IgA antibodies (by ELISA) have been superseded by these more sensitive tests; has S/S = 80%/80–90%. IgA antigliadin antibodies become undetectable 3–6 months after gluten abstinence; may be used to monitor dietary compliance. May be most effective marker for children <3 years of age. Gliadin is a component of gluten. False-negative results may occur in patients on immunosuppressive therapy. If patient is IgA deficient, serology using IgG-tTG or IgG-EMA should be used.
Molecular tests
: HLA variation DQ2 is expressed in approximately 95% of patients; HLA-DQ8 performed by DNA testing is expressed in approximately 5% of patients; absence of these virtually excludes this diagnosis.
Gluten challenge
: No longer considered essential to establish the diagnosis. It is done if the diagnosis is uncertain and not documented by biopsy before gluten withdrawal, to determine if symptoms and mucosal changes occur.
Xylose tolerance test
: Distinguishes malabsorption caused by impaired transport across diseased mucosa from that caused by impaired digestion in the lumen. Normal in many patients with mild to moderate disease not usually performed.
Considerations
Firm diagnosis requires definite clinical response to gluten-free diet in 3–9 months, preferably with histologic documentation that the mucosa has reverted to normal by repeat biopsy. If the patient fails to respond to rigid dietary control, biopsy should be repeated to rule out GI lymphoma, giardiasis, hypogammaglobulinemia, and other causes of villous atrophy, as well as diet should be rechecked.
Malabsorption may cause folate deficiency with megaloblastic bone marrow and iron deficiency with mild hypochromic macrocytic anemia. Celiac disease should always be considered in cases of iron deficiency or macrocytic anemia. May also have coagulopathy due to vitamin K deficiency and hypo-calcemia and vitamin D deficiency causing osteomalacia. In patients with unexplained diarrhea or malabsorption, celiac sprue should be ruled out by small bowel biopsy.
Laboratory findings due to frequently associated autoimmune diseases (e.g., thyroid, liver, type 1 DM, dermatitis herpetiformis [≤20% of celiac patients], Addison disease, arthritis) and other diseases (e.g., selective IgA deficiency; hyposplenism, T-cell lymphoma of the small intestine; also Down syndrome, IgA nephropathy, IBD). Patients who should be screened include those with steatorrhea, malabsorption, or autoimmune diseases.
Suggested Readings
Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review.
Gastroenterol Hepatol.
2007;3:112–122.
Farrell RJ, Kelly CP. Celiac sprue.
N Engl J Med.
2002;346:180–188.
Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland.
N Engl J Med.
2003;348:2517–2524.
ENTEROPATHY, PROTEIN LOSING
Definition