Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (607 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

Allergen challenge tests: A nasal challenge can be used to test specific as well as nonspecific reactivity. It is clinically impractical and rarely performed.
Other tests: Nasal cytology is performed by some to help differentiate rhinitis due to allergy from that due to infection. Wright stain of nasal secretions usually, but not always, reveals predominance of eosinophils in cases of allergic rhinitis. The presence of neutrophils suggests an infectious process. Other diagnostic testing assays, cytotoxic testing, provocation neutralization testing, and specific or nonspecific IgG determinations are unproven and inappropriate.
Component-resolved diagnosis (CRD) is also known as molecular allergy diagnosis, in which individual allergen molecules are used to characterize a patient’s IgE specificity. This offered either as panels of selected allergens that can be used as serum (ImmunoCAP) assays or microarray-based assay of more than 100 molecules. CRDs are available in Europe and used only as a research tool in the United States.
Suggested Readings
Gentile D, Bartholow A, Valovirta E, et al. Current and future directions in pediatric allergic rhinitis.
J Allergy Clin Immunol: In Practice.
2013;1:214–226.
Ng ML, Warlow RS, Chrishanthan N, et al. Preliminary criteria for the definition of allergic rhinitis: a systemic evaluation of clinical parameters in a disease cohort (I).
Clin Exp Allergy.
2000;30:1314.
Ng ML, Warlow RS, Chrishanthan N, et al. Preliminary criteria for the definition of allergic rhinitis: a systemic evaluation of clinical parameters in a disease cohort (II).
Clin Exp Allergy.
2000;30:1417.
ACID–BASE DISORDERS
Definition
Acid–base balance disorders are usually encountered in acutely ill or complicated medical and surgical patients. The plasma concentration of hydrogen ion (pH
+
) is very low (approximately 40 nmol/L), and it is constantly maintained within a narrow range by
Excretion of CO
2
by the lungs
Excretion of H
+
by the kidneys
Everyday approximately 15,000 mmol of CO
2
is produced by endogenous metabolism and then excreted by the lungs. Similarly, normal diet generates 50–100 mmol of H
+
per day, derived mostly from metabolism of sulfur-containing amino acids. The maintenance of stable H
+
level is required for normal cellular function, since small fluctuations in the H
+
concentrations have important effects on the activity of cellular enzymes. There is a relatively narrow range of extracellular H
+
concentration (16–160 nmol/L: pH 7.8–6.8) that is compatible with life. Changes in H
+
are nonlinear; hence, measuring pH masks the magnitude of acid–base disorders.
BUFFER SYSTEMS (BICARBONATE–CARBONIC ACID)
This buffer is at highest concentration in the blood, and it is also plays an important role in acid–base regulation. Carbonic acid (CO
2
) is a volatile acidic gas and is soluble in water. It readily diffuses from cells to blood, where it combines with water to produce carbonic acid, which immediately dissociates in to bicarbonate and hydrogen ions.
pH, HCO
3
−
, and pCO
2
are related by the equation:
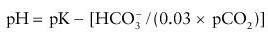
where pK is defined as the pH at which HCO
3
−
and H
2
CO
3
(0.03% pCO
2
) are in equal concentrations.
Normal concentrations of HCO
3
−
and H
2
CO
3
in the blood are in the ratio of 20:1 and with a pK of 6.1. This excess base HCO
3
−
, along with volatility of CO
2
, gives ability to prevent overaccumulation of acid. The lungs through the loss of CO
2
provide the ultimate buffering capacity. Bicarbonate is regulated by the kidneys and CO
2
is regulated by the lungs and the ratio of HCO
3
−
to H
2
CO
3
that determines the pH.