Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (608 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

BOOK: Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis
2.06Mb size Format: txt, pdf, ePub
Laboratories measure pH and pCO
2
directly and calculate HCO
3
using the Henderson-Hasselbalch equation:
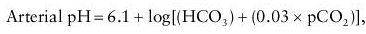
where 6.1 is the dissociation constant for CO
2
in aqueous solution and 0.03 is a constant for the solubility of CO
2
in plasma at 37°C.
RESPIRATORY AND METABOLIC SYSTEMS IN ACID–BASE REGULATION
Respiratory System
Arterial CO
2
is influenced by ventilatory rate; pCO
2
is considered the respiratory component of the bicarbonate–CO
2
buffer system. Because CO
2
is the end product of aerobic metabolism, continuous buffering CO
2
is required for the regulation of pH.
The arterial pCO
2
represents a balance between tissue production of CO
2
and pulmonary removal of CO
2
. An elevated pCO
2
usually indicates hyperventilation. This leads to respiratory acidosis (hypoventilation) or respiratory alkalosis (hyperventilation).
The respiratory rate can alter arterial pH in minutes.
Metabolic (Renal) System
When H
+
levels deviated from normal, the kidneys respond by reabsorbing or secreting hydrogen, bicarbonate, and other ions to regulate the blood pH. Metabolic acidosis may develop; either H
+
accumulates or bicarbonate ions are lost. Metabolic alkalosis may develop from either loss of H
+
or increase in bicarbonate.
Unlike the respiratory system, the renal system requires hours to days to significantly affect pH by altering the excretion of bicarbonate.
ANALYZING ACID–BASE DISORDERS (Table
13-2
)
When analyzing acid–base disorders, several points should be kept in mind:
Determination of pH and blood gases should be performed preferentially on arterial blood. Venous blood is useless for judging oxygenation or if perfusion is not adequate, but it offers an estimate of acid–base status. Venous pH is approximately 0.03−0.04 lower than in arterial blood, and CO
2
pressure (pCO
2
) is normally approximately 3–4 mm higher.
Blood specimens should be packed in ice immediately; a delay of even a few minutes will cause erroneous results, especially if the WBC count is high.
Other books
Ghoul Interrupted by Victoria Laurie
Egg-Drop Blues by Jacqueline Turner Banks
Secret Confessions: Down & Dusty — Skye by Rhyll Biest
First Round Lottery Pick by Franklin White
Darling obstacles by Boswell, Barbara, Copyright Paperback Collection (Library of Congress) DLC
CHEAP SMUT: Four Erotic Romance Novels (Boxed Set) by Scott Hildreth
Gemini Rain by Lj McEvoy
Fire by Deborah Challinor
Damage Control by Robert Dugoni
Dark Gold by Christine Feehan